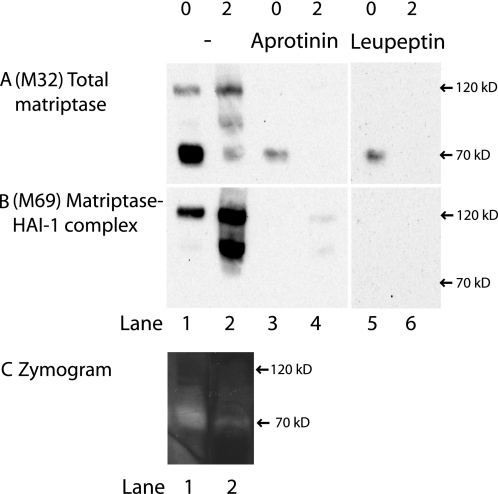
FIGURE 5.
Matriptase is active on the basolateral plasma membrane but not intracellularly. Caco-2 cells on Transwell filters were surface-biotinylated from the basolateral side at 4 °C. Some were lysed immediately after biotinylation (0) and some were incubated for 2 h at 37 °C to transform all biotin-labeled matriptase into activated matriptase in complex with HAI-1 (2). Biotinylated proteins were precipitated with monomeric avidin and gently eluted with biotin. The avidin pull-downs were divided into three groups: No further treatment (lanes 1 and 2), pull-down with aprotinin-Sepharose (lanes 3 and 4) and pull-down with leupeptin-Sepharose (lanes 5 and 6). The samples were analyzed by SDS-PAGE and Western blot with antibodies against total matriptase (M32) and matriptase-HAI-1 complex (M69). A, only at time 0 was it possible to pull-down M32-detectable matriptase with both aprotinin and leupeptin. B, M69-detectable matriptase was pulled down with the monomeric avidin (B, lanes 1 and 2), but was lost with additional inhibitor pull-down (B, lanes 3–6). C, two avidin-purified fractions showed gelatinolytic properties with a band around 70 kDa (C, lanes 1 and 2), matching the size and pattern of A, lanes 1 and 2. Results shown are representative of two independent experiments.