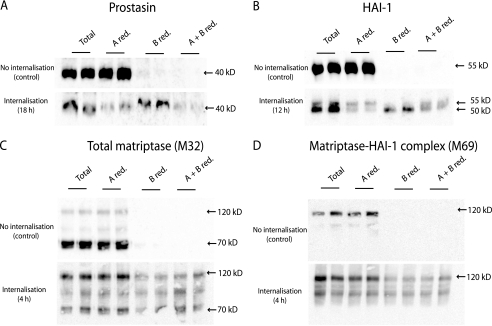
FIGURE 7.
Prostasin and HAI-1 are transcytosed from the basolateral to the apical plasma membrane. Caco-2 cells were surface biotinylated from the basolateral side at 4 °C. The labeled cells were incubated at 37 °C for the time indicated in the internalization panel (4, 12, or 18 h as indicated). After incubation the proteins remaining on the plasma membrane were biotin-stripped, using the membrane non-permeant reducing agent glutathione, from either apical (A red.), basolateral (B red.), or both sides (A + B red.). Biotin reduction was omitted in a set of samples to monitor the total amount of biotinylated protein remaining after the incubation (Total). The experiment was performed in duplicates. Biotinylated proteins were precipitated with monomeric avidin agarose, separated with SDS-PAGE and analyzed by Western blotting. A, prostasin could be biotin-stripped from the apical side (A red.) after 18 h of internalization, as a decrease in signal was observed compared with the total samples. This shows that prostasin has moved from the basolateral to the apical side. When surface proteins were biotin-stripped from the basolateral side no decrease in signal was observed (B red.), once again showing that no biotinylated prostasin was left on the basolateral side after 18 h. To clarify if some of the prostasin was located intracellularly, the cells were biotin stripped from both apical and basolateral side (A + B red.). Biotin-stripping from both apical and basolateral side removed the majority of the biotinylated prostasin suggesting that all of prostasin had within the 18 h transferred from the basolateral to the apical plasma membrane. B, HAI-1 was transcytosed from the basolateral to the apical plasma membrane within 12 h. Most of HAI-1 could be biotin stripped from the apical side after incubation (A red.) where a major fraction was left after basolateral reduction (B. red) showing that a large fraction of biotinylated HAI-1 had moved from the basolateral to the apical plasma membrane. It was demonstrated that the faster migrating form of HAI-1 resides at the apical plasma membrane 12 h after biotinylation and the slower migrating form remained at the basolateral plasma membrane, as these could be biotin-stripped from their respective sites (A red. compared with B red., respectively). C, after just 4 h of incubation most biotinylated matriptase was no longer detectable in the cell. The remaining matriptase was mainly found in the 120 kDa complex with HAI-1. A fraction of the matriptase-HAI-1 complex could be biotin stripped from the basolateral side (B red.) but not the apical side (A red.). D, M69 detectable matriptase showed the same amount of matriptase-HAI-1 complex at time 0 as for 4 h of incubation. A fraction of the complex was reducible from the basolateral side after 4 h of incubation (B red.). Results shown are representative of three independent experiments.