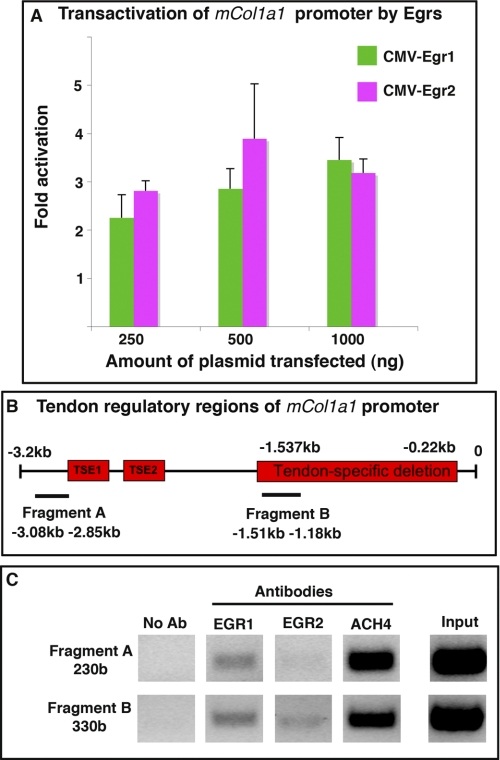
FIGURE 8.
Egr1 and Egr2 enhance the transcriptional activity of the mouse Col1a1 proximal promoter and are recruited to tendon regulatory regions. A, NIH/3T3 cells were transiently co-transfected with the reporter construct pJ320, containing a 3.2-kb region of the Col1a1 promoter including tendon-specific elements, cloned upstream of the lacZ gene, and with different amounts of CMV-Egr2, CMV-Egr1, or CMV control. Transfection of CMV-Egr1 or CMV-Egr2 had a trans-activating effect on pJ320. Results were expressed as mean and S.E. B, schematic representation of the 3.2-kb Col1a1 proximal promoter. The tendon-specific elements, TSE1 and TSE2, located around −2.8 and −2.3 kb and the region between −1.537 and −0.22 kb required for tendon specific expression of Col1a1 (named tendon-specific deletion) are represented by red boxes. Horizontal lines indicate the amplicon positions (not to scale) used for the ChIP assay. Fragment A is a 230-base region (−3.08 to −2.85 kb) flanking TSE1 and TSE2, and fragment B is a 330-base region (1.51 to −1.18 kb), included into the tendon-specific deletion (−1.537 to −0.22 kb). C, ChIP assays were performed on hind limbs from E18.5 mice with antibodies against EGR1, EGR2, or AcH4, (acetylated histone H4), as a positive control or without an antibody (no Ab) as a negative control. ChIP products were analyzed by PCR, to study the interaction of each EGR with tendon regulatory regions of mouse Col1a1 promoter. Primers targeting fragment A identify DNA regions immunoprecipitated by EGR1, but not by EGR2, whereas primers targeting fragment B identify DNA regions immunoprecipitated by EGR1 and EGR2.