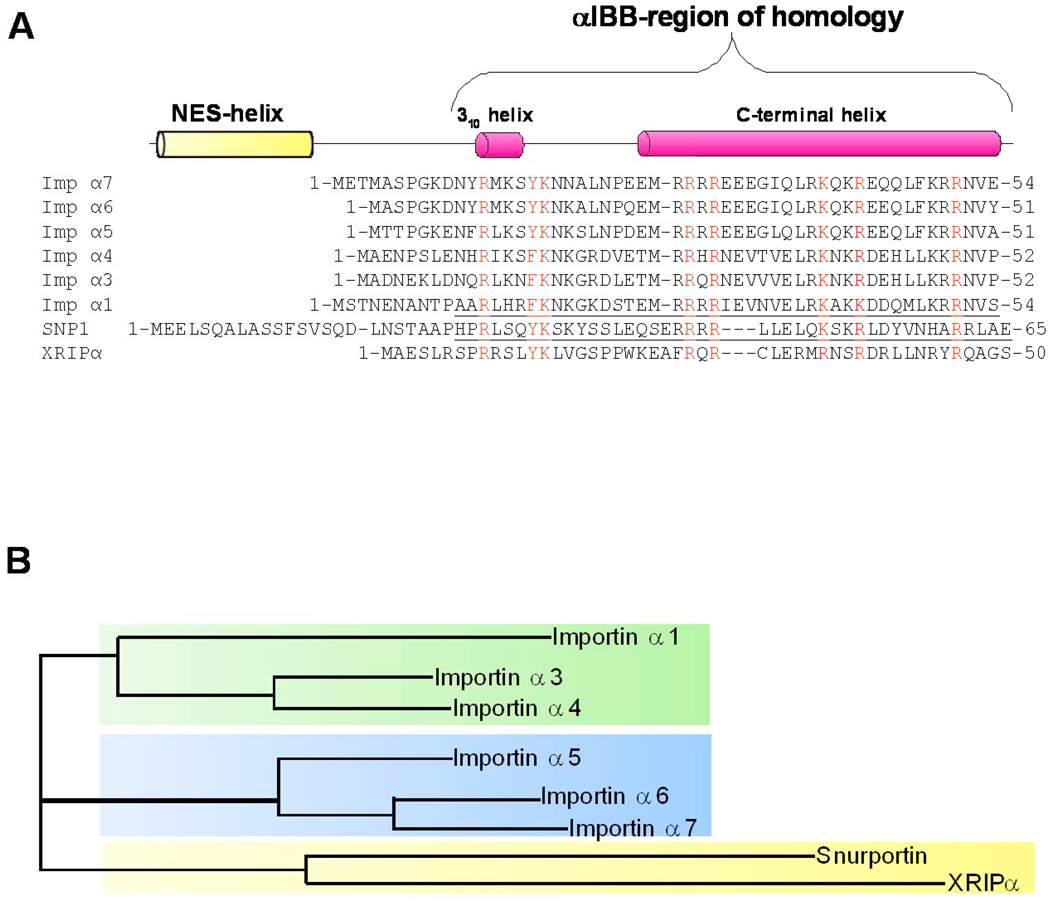
Fig. 2. Evolution and conservation of IBB-domains in adaptor proteins.
(A) Sequence alignment of IBB-domains found in six human isoforms of importin α (αl, α3, α4, α5, α6, α7), human snurportin (SNP1), and human XRIPα. A schematic representation of the IBB-secondary structure is drawn above the sequence alignment. In the alignment, highly conserved basic residues are colored in red. The underlined sequences refer to the IBB-domains that were crystallized in complex with importin β, namely importin αl IBB-domain (res. 11–54) [9] and snurportin IBB-domain (res. 25–65) [10]. Notice that the N-terminal extension comprising the NES [56] is conserved only in sIBB, and in other snurportin homologues [10], but not in αIBBs and xXRIPα. (B) Phylogenetic tree of human IBB-domain sequences shown in panel (A). In the tree, the branch lengths are proportional to the predicted evolutionary time between sequences. Three subfamilies of IBBs (shaded in green, blue and yellow) are identified. Both sequence alignment and phylogenetic tree were generated using the program ClustalW [118].