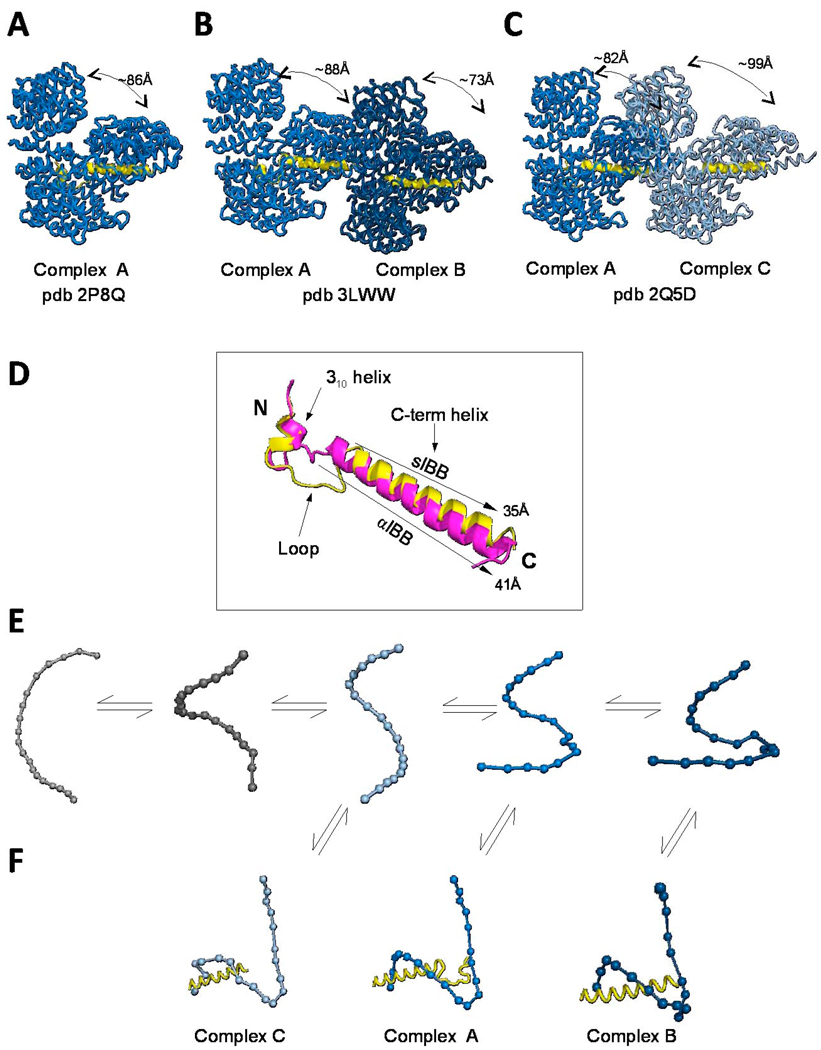
Fig. 4. Structure and recognition of IBB-domains by importin β.
Structural views of importin β bound to the sIBB (res 25–65) in three distinct crystal forms. (A) 2.35Å resolution structure (pdb 2P8Q) of importin β bound to sIBB (res. 25–65) containing one complex in the asymmetric unit. This structure represents the strained conformation of the protein. (B) 3.15Å resolution structure (pdb 3LWW) with two importin β/sIBB complexes (indicated as A–B) in the same asymmetric unit. Complex A is identical to the high resolution structure in panel (A). In contrast, complex B presents an ultra-strained conformation of importin β (C) 3.2Å resolution structure (pdb 2Q5D) with two importin β/sIBB (res. 25–65) complexes (indicated as A–C) in the same asymmetric unit. Complex A is equivalent to importin β/sIBB complex A in panel (A) and (B), while complex C has a dramatically less strained conformation of importin β. All structures in (A–C) are aligned using importin β in complex A as reference. In all complexes, the sIBB is colored in yellow and the distance between N- and C-terminal HEAT repeats of importin β is indicated. (D) Ribbon diagram of sIBB superimposed onto the αIBB domain (in yellow and magenta, respectively). Both IBBs were crystallized in complex with full length human importin β (pdb 2P8Q and 1QGK, respectively). The sIBB α-helix is ∼5 Å shorter than the αIBB C-terminal α-helix due to a three amino acid gap in its amino acid sequence between residues 46–47 (Fig. 2A). (E) Population selection model proposed for the recognition of sIBB by importin β. Putative conformers of importin β are shown as beads-on-string, where each HEAT repeat is represented by a sphere. This view emphasizes the different degree of intramolecular opening in different importin β conformers. Grey molecules are conformations of importin β not observed crystallographically, but likely seen by small angle X-ray scattering [72]. (F) Stabilization of different conformers by the sIBB-domain is thought to populate importin β conformers, which can be crystallized and hence trapped in a crystal lattice [75].