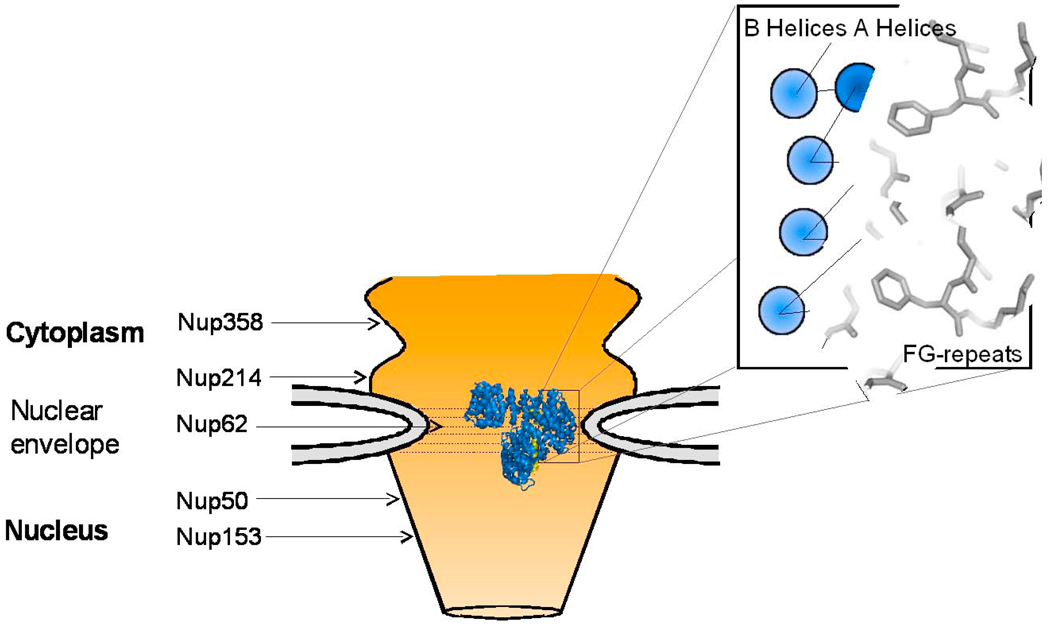
Fig. 5. Role of IBB-domain in translocation of import complexes through the NPC.
Structure of the importin β/sIBB complex (colored in blue and yellow, respectively) at the central channel of the NPC, which is formed primarily by Nup62 complex [119]. This schematic is not to scale: the structure of importin β is greatly magnified with respect to the NPC central channel. Key FG-nucleoporins are indicated respective to their location in the NPC. In the blowup is cartoon of a region of the importin β HEAT repeats bound to FG-motifs. HEAT repeats are represented as circles, with the outer A helices in dark blue, and the concave B helices as light blue. B helices are responsible for binding to the IBB-domain while the convex surface interacts with the nucleoporins. Phenylalanine side chains emanating from FG-nups are modeled interacting with the groove in between A helices of importin β.