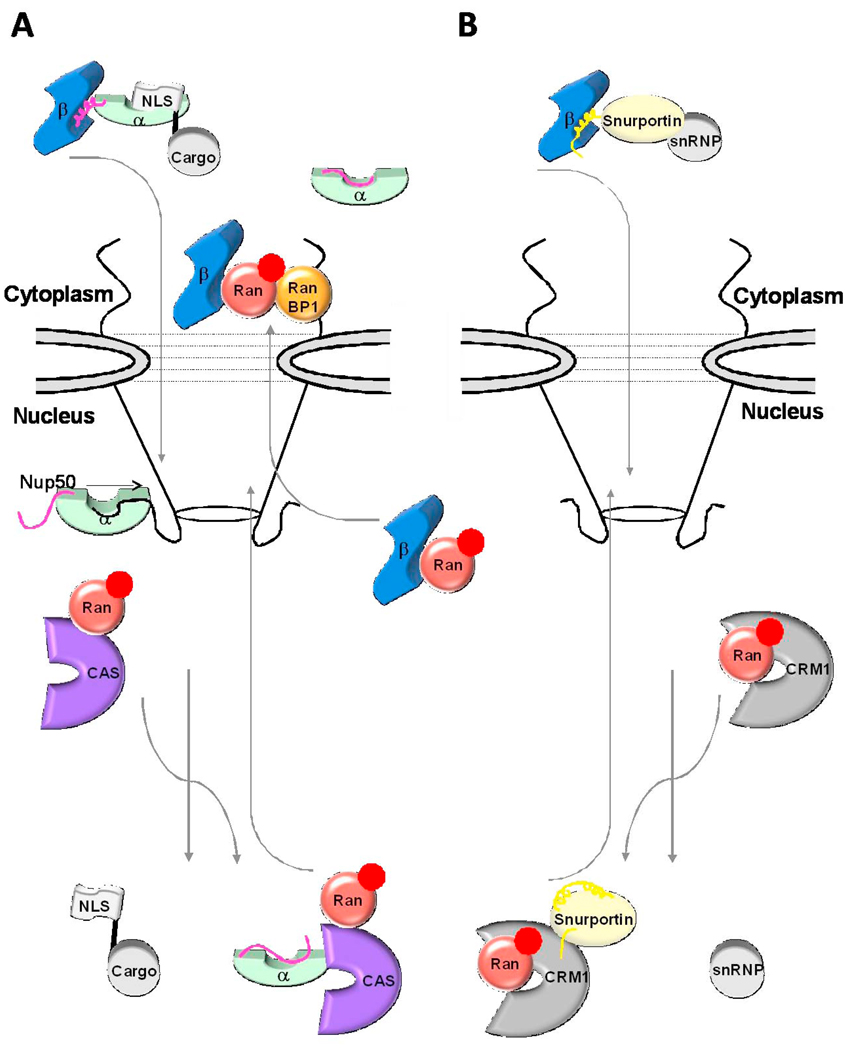
Fig. 6. Role of IBB-domain in recycling of adaptors and importin β to the cytoplasm.
(A) Cartoon diagram of the importin β/α/NLS-cargo complex (in blue, green, and grey, respectively) assembled in the cytoplasm. Upon nuclear entry RanGTP (in red) displaces importin β, and the nucleoplasmic nucleoporins Nup50 (black line) binds importin α NLS-groove and displaces the NLS-cargo. The export complex of CAS and RanGTP (shown in purple and red, respectively) displaces Nup50 from importin α and with the αIBB locked in an autoinhibited position an export-competent forms to be recycled to the cytoplasm. (B) An analogous scenario for the importin β/snurportin/snRNP import complex (in blue, yellow and grey, respectively). Upon nuclear entry the N-terminal seventeen amino acids of the sIBB binds to the export factor CRM1 (shown in silver). Colored in dark yellow is the remainder of the sIBB not involved in interactions with CRM1, shown as two surface exposed helices separated by a kinked loop.