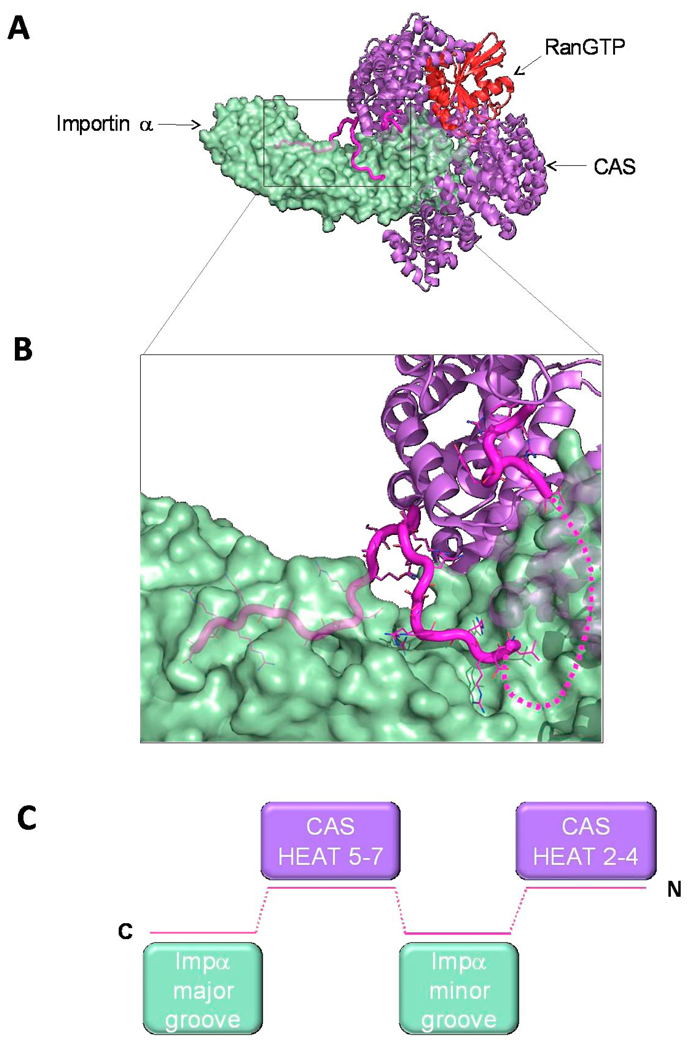
Fig. 7. Structure of the αIBB in the importin α/CAS/RanGTP export complex.
(A) Structure of the trimeric importin α export complex (pdb 1WA5) [78], which consists of Cse1p (yeast homologue of CAS), RanGTP and Kap60p (yeast homologue of importin α), in purple, red and green, respectively. The IBB-domain is colored in magenta. (B) Zoom in view of αIBB interaction with Cse1p and the NLS binding grooves of importin α. IBB residues 20–31 were not observed in the crystal structure and are modeled as a dashed line. (C) Schematic representation of the αIBB emphasizing the complex binding interactions of IBB-domain (magenta) with Cse1p (purple) and the NLS binding sites on importin α (green).