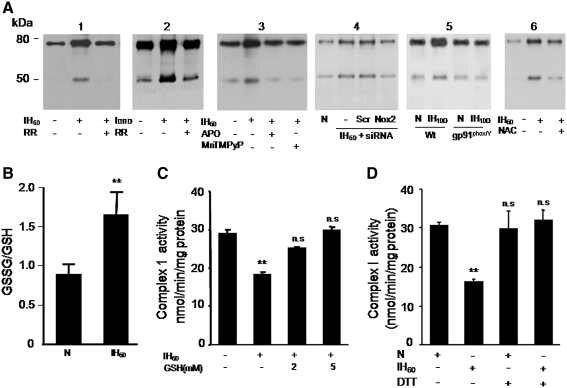
FIG. 5.
IH increases S-glutathionylation of mitochondrial complex I subunits. (A) Representative immunoblots showing increased S-glutathionylation of 75- and 55-kDa subunits of the complex I in IH60 and ionomycin (Iono; 1.4 μM)-treated PC12 cells and blockade of the responses by RR (10 μM; panels 1 and 2). Blockade of IH60-induced S-glutathionylation of complex I subunits by Nox inhibitor apocynin (Apo; 500 μM) and antioxidant (MnTMPyP; 50 μM; panel 3) and by Nox2 siRNA (panel 4). S-glutathionylation of complex I subunits in brain stem cell lysates from wild-type (Wt) and gp91phox-/Y mice treated with either 10 days of normoxia or IH (panel 5). Note the absence of increased S-glutathionylation of complex I subunits in tissues lysates from IH-treated gp91phox-/Y mice. Blockade of IH60-induced S-glutathionylation of complex I subunits by N-acetyl-cysteine (2 mM NAC), a precursor of glutathione (panel 6). (B) Ratio of oxidized from of glutathione/GSH was determined in normoxic (N) and IH60-treated PC12 cells as described in Materials and Methods. (C) IH-induced inhibition of complex I activity is prevented in the presence of 2 and 5 mM GSH or (D) 2 μM dithiothreitol (DTT). Data represent the mean ± SEM from three to five independent experiments **p < 0.01 compared to normoxic controls (N). GSH, reduced form of glutathione; MnTMPyP, manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin; NAC, N-acetyl-cysteine.