Abstract
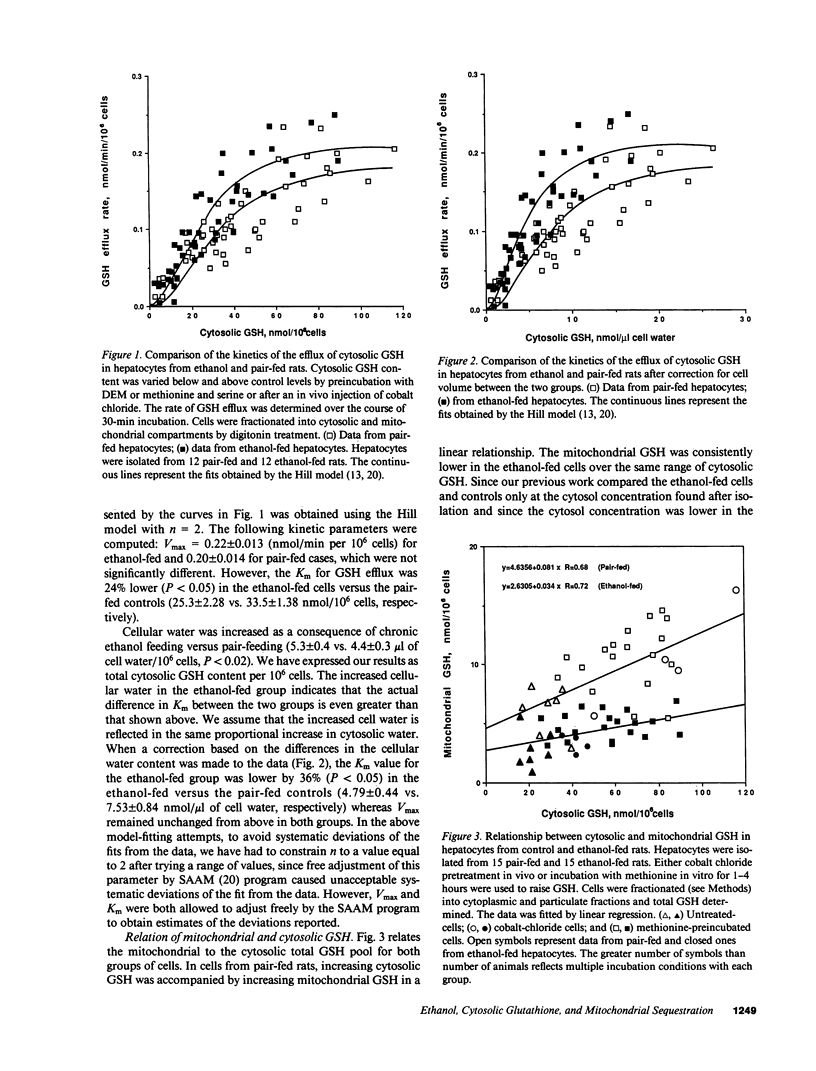
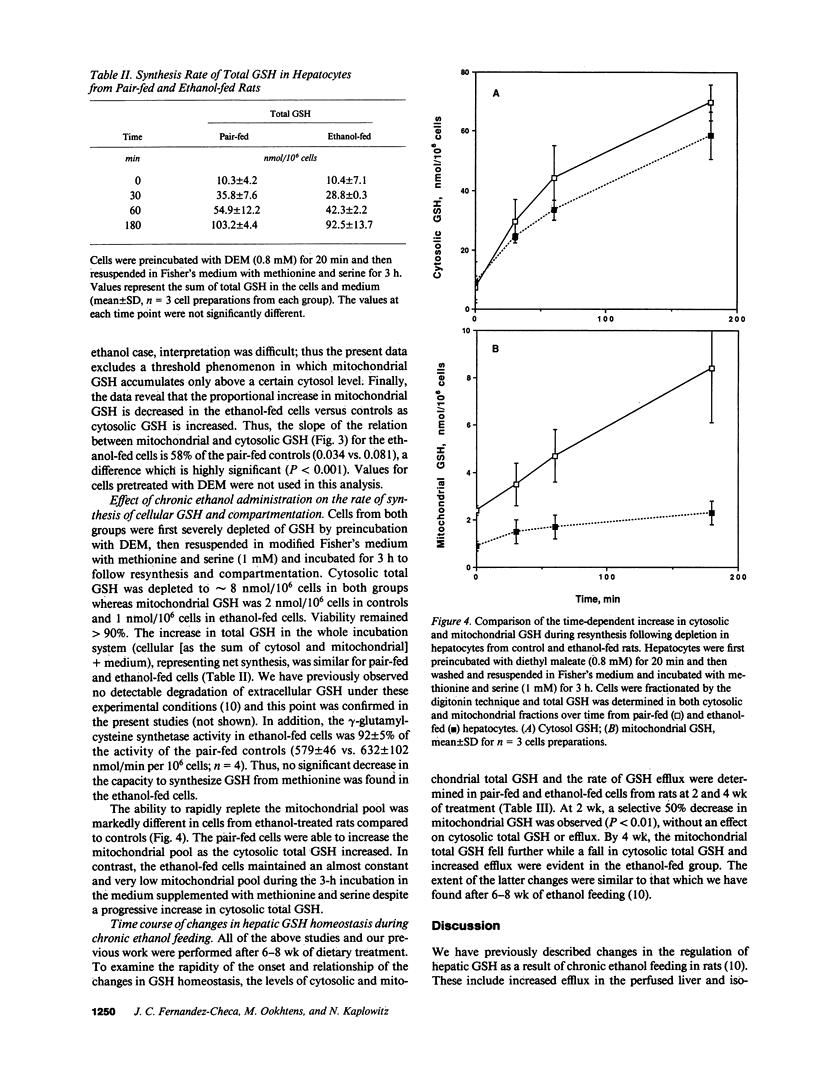
Chronic ethanol feeding to rats increases the sinusoidal component of hepatic glutathione (GSH) efflux, despite a lower steady-state GSH pool size. In the present studies, no increase of biliary GSH efflux in vivo was found in chronic ethanol-fed cells. Studies were performed on ethanol-fed and pair-fed cells to identify the kinetic parameters of cellular GSH concentration-dependent efflux. The relationship between cytosolic GSH and the rate of efflux was modeled by the Hill equation, revealing a similar Vmax, 0.22 +/- 0.013 vs. 0.20 +/- 0.014 nmol/min per 10(6) cells for ethanol-fed and pair-fed cells, respectively, whereas the Km was significantly decreased (25.3 +/- 2.3 vs. 33.5 +/- 1.4 nmol/10(6) cells) in ethanol-fed cells. The difference in Km was larger when the data were corrected for the increased water content in ethanol-fed cells. We found a direct correlation between mitochondria and cytosolic GSH, revealing that mitochondria from ethanol-fed cells have less GSH at all cytosolic GSH values. The rate of resynthesis in depleted ethanol-fed cells in the presence of methionine and serine was similar to control cells and gamma-glutamylcysteine synthetase remained unaffected by chronic ethanol. However, the reaccumulation of mitochondrial GSH as the cytosolic pool increased was impaired in the ethanol cells. The earliest time change in GSH regulation was a 50% decrease in the mitochondrial GSH at 2 wk.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott W. A., Meister A. Intrahepatic transport and utilization of biliary glutathione and its metabolites. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1246–1250. doi: 10.1073/pnas.83.5.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw T. Y., Ookhtens M., Kaplowitz N. Inhibition of glutathione efflux from isolated rat hepatocytes by methionine. J Biol Chem. 1984 Aug 10;259(15):9355–9358. [PubMed] [Google Scholar]
- Aw T. Y., Ookhtens M., Kaplowitz N. Mechanism of inhibition of glutathione efflux by methionine from isolated rat hepatocytes. Am J Physiol. 1986 Sep;251(3 Pt 1):G354–G361. doi: 10.1152/ajpgi.1986.251.3.G354. [DOI] [PubMed] [Google Scholar]
- Aw T. Y., Ookhtens M., Kuhlenkamp J. F., Kaplowitz N. Trans-stimulation and driving forces for GSH transport in sinusoidal membrane vesicles from rat liver. Biochem Biophys Res Commun. 1987 Feb 27;143(1):377–382. doi: 10.1016/0006-291x(87)90676-0. [DOI] [PubMed] [Google Scholar]
- Aw T. Y., Ookhtens M., Ren C., Kaplowitz N. Kinetics of glutathione efflux from isolated rat hepatocytes. Am J Physiol. 1986 Feb;250(2 Pt 1):G236–G243. doi: 10.1152/ajpgi.1986.250.2.G236. [DOI] [PubMed] [Google Scholar]
- Ballatori N., Jacob R., Boyer J. L. Intrabiliary glutathione hydrolysis. A source of glutamate in bile. J Biol Chem. 1986 Jun 15;261(17):7860–7865. [PubMed] [Google Scholar]
- Booth J., Boyland E., Sims P. An enzyme from rat liver catalysing conjugations with glutathione. Biochem J. 1961 Jun;79(3):516–524. doi: 10.1042/bj0790516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Fraga C. G., Varsavsky A. I., Koch O. R. Increased chemiluminescence and superoxide production in the liver of chronically ethanol-treated rats. Arch Biochem Biophys. 1983 Dec;227(2):534–541. doi: 10.1016/0003-9861(83)90482-4. [DOI] [PubMed] [Google Scholar]
- Fernandez-Checa J. C., Ookhtens M., Kaplowitz N. Effect of chronic ethanol feeding on rat hepatocytic glutathione. Compartmentation, efflux, and response to incubation with ethanol. J Clin Invest. 1987 Jul;80(1):57–62. doi: 10.1172/JCI113063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Checa J. C., Ren C., Aw T. Y., Ookhtens M., Kaplowitz N. Effect of membrane potential and cellular ATP on glutathione efflux from isolated rat hepatocytes. Am J Physiol. 1988 Oct;255(4 Pt 1):G403–G408. doi: 10.1152/ajpgi.1988.255.4.G403. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Origin and turnover of mitochondrial glutathione. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplowitz N., Eberle D. E., Petrini J., Touloukian J., Corvasce M. C., Kuhlenkamp J. Factors influencing the efflux of hepatic glutathione into bile in rats. J Pharmacol Exp Ther. 1983 Jan;224(1):141–147. [PubMed] [Google Scholar]
- Ku R. H., Billings R. E. The role of mitochondrial glutathione and cellular protein sulfhydryls in formaldehyde toxicity in glutathione-depleted rat hepatocytes. Arch Biochem Biophys. 1986 May 15;247(1):183–189. doi: 10.1016/0003-9861(86)90547-3. [DOI] [PubMed] [Google Scholar]
- LaBaume L. B., Merrill D. K., Clary G. L., Guynn R. W. Effect of acute ethanol on serine biosynthesis in liver. Arch Biochem Biophys. 1987 Aug 1;256(2):569–577. doi: 10.1016/0003-9861(87)90614-x. [DOI] [PubMed] [Google Scholar]
- Meredith M. J., Reed D. J. Depletion in vitro of mitochondrial glutathione in rat hepatocytes and enhancement of lipid peroxidation by adriamycin and 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU). Biochem Pharmacol. 1983 Apr 15;32(8):1383–1388. doi: 10.1016/0006-2952(83)90451-3. [DOI] [PubMed] [Google Scholar]
- Meredith M. J., Reed D. J. Status of the mitochondrial pool of glutathione in the isolated hepatocyte. J Biol Chem. 1982 Apr 10;257(7):3747–3753. [PubMed] [Google Scholar]
- Moldéus P., Högberg J., Orrenius S. Isolation and use of liver cells. Methods Enzymol. 1978;52:60–71. doi: 10.1016/s0076-6879(78)52006-5. [DOI] [PubMed] [Google Scholar]
- Ookhtens M., Hobdy K., Corvasce M. C., Aw T. Y., Kaplowitz N. Sinusoidal efflux of glutathione in the perfused rat liver. Evidence for a carrier-mediated process. J Clin Invest. 1985 Jan;75(1):258–265. doi: 10.1172/JCI111682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookhtens M., Lyon I., Fernandez-Checa J., Kaplowitz N. Inhibition of glutathione efflux in the perfused rat liver and isolated hepatocytes by organic anions and bilirubin. Kinetics, sidedness, and molecular forms. J Clin Invest. 1988 Aug;82(2):608–616. doi: 10.1172/JCI113639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson J. L., Mitchell M. C. Increased hepatic efflux of glutathione after chronic ethanol feeding. Biochem Pharmacol. 1986 May 1;35(9):1533–1537. doi: 10.1016/0006-2952(86)90121-8. [DOI] [PubMed] [Google Scholar]
- Reed D. J., Babson J. R., Beatty P. W., Brodie A. E., Ellis W. W., Potter D. W. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980 Jul 15;106(1):55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- Reed D. J. Regulation of reductive processes by glutathione. Biochem Pharmacol. 1986 Jan 1;35(1):7–13. doi: 10.1016/0006-2952(86)90545-9. [DOI] [PubMed] [Google Scholar]
- Seelig G. F., Meister A. Gamma-glutamylcysteine synthetase from erythrocytes. Anal Biochem. 1984 Sep;141(2):510–514. doi: 10.1016/0003-2697(84)90079-4. [DOI] [PubMed] [Google Scholar]
- Shaw S., Jayatilleke E., Ross W. A., Gordon E. R., Leiber C. S. Ethanol-induced lipid peroxidation: potentiation by long-term alcohol feeding and attenuation by methionine. J Lab Clin Med. 1981 Sep;98(3):417–424. [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]


