Abstract
Conscious awareness of objects in the visual periphery is limited. This limit is not entirely the result of reduced visual acuity, but is primarily caused by crowding—the inability to identify an object when surrounded by clutter. Crowding represents a fundamental limitation of the visual system, and has to date been unexplored in infants. Do infants have a fine-grained “spotlight”, similar to adults, or a diffuse “lantern” that sets limits on what they can register in the periphery? An eye-tracking paradigm was designed to psychophysically measure crowding in 6- to 15-month-olds by showing pairs of faces at three eccentricities, in the presence or absence of flankers, and recording infants’ first saccade from central fixation to either face. Results reveal that infants can discriminate faces in the periphery, and flankers impair this ability as close as 3 degrees; the effective spatial resolution of visual perception increased with age but was only half that of adults.
Keywords: inversion, crowding, attention, peripheral vision, Mooney face
Conscious vision in the peripheral visual field is severely limited. This limit is not entirely due to decreased acuity; it is more fundamentally caused by crowding—the reduced ability to identify individual objects as a result of surrounding clutter (Levi, 2008; Pelli & Tillman, 2008). Phenomenologically, crowding causes an easily recognized peripheral object to appear as a jumbled mass of unbound features (Figure 1); observers recognize that there is “stuff” there, but cannot identify the particular object. Psychophysically measuring the effect of crowding in the periphery provides a means of estimating the size of the “spotlight”, or window, which defines the spatial resolution of visual awareness. Crowding occurs any time multiple objects fall within this window. This limit has been well characterized in adults, but is completely unknown in infants. It is important to examine these limits in infants, however, because understanding the spatial resolution of visual perception in infants is a prerequisite for understanding the development of visual attention, object recognition, and visually-guided action.
Figure 1.

A demonstration of crowding. While fixating on the cross, it is extremely difficult to identify the middle letter in a string of peripheral letters (top panel), however it is easy to identify the middle letter when it is presented in isolation (middle panel). This reveals that the limit of adult spatial resolution is not due to reduced acuity; rather, it is due to crowding.
Although the spatial resolution of visual awareness has not been measured in infants, peripheral visual acuity has been tested to a limited extent. Research with infants has typically evaluated the direction and latency of saccadic eye movements in response to the detection of a peripheral stimulus and has shown that visual fields are quite narrow during the first months of life (approximately 30 degrees of visual angle (deg)), becoming progressively wider after 5 months (60 deg) and 1 year (80–90 deg), with slow growth toward adult levels (de Schonen, McKenzie, Maury, & Bresson, 1978; Maurer & Lewis, 1979; Sireteanu, Fronius, & Constantinescu, 1994). Previous studies have also shown that infants can discriminate stimuli in the periphery on the basis of broad features including size, color, and shape (Cohen, 1972; Maurer & Lewis, 1979; Salapatek, 1975). These studies might predict that infants have a more limited “spotlight” of visual awareness compared to adults, or perhaps that they have more of a “lantern” of visual consciousness (Gopnik, 2009), which may limit the resolution with which information is accessed. The grain of conscious peripheral vision in infants therefore remains an open developmental question.
Crowding offers a means of measuring the resolution of visual perception in the periphery. In adults, crowding has been demonstrated with gratings (Andriessen & Bouma, 1976), numbers (Strasburger, Harvey, & Rentschler, 1991), letters (Townsend, Taylor, & Brown, 1971), and faces (Farzin, Rivera, & Whitney, 2009; Louie, Bressler, & Whitney, 2007; Pelli, Palomares, & Majaj, 2004), among other stimuli. In crowded scenes, the spatial resolution of perception is reduced proportionally to eccentricity and flanker density; the window within which crowding occurs extends as far as half the retinal eccentricity of the target in normal adult peripheral vision (Bouma’s law), and only 1 degree or less in normal foveal vision (Bouma, 1970; Levi, 2008; Pelli & Tillman, 2008).
Current evidence strongly supports the theory that multiple mechanisms and levels of crowding exist in adults. One model proposes that the inability to identify a crowded target item in the periphery is the result of interference between low-level elementary features within the same receptive field (Flom, Heath, & Takahashi, 1963; Kooi, Toet, Tripathy, & Levi, 1994) or excessive feature binding within an “integration field” (Pelli, et al., 2004). Another explanation is that crowding is the result of a higher-level limit imposed on the resolution of spatial attention (He, Cavanagh, & Intriligator, 1997; Intriligator & Cavanagh, 2001). Regardless of the particular mechanisms that contribute to crowding, it is generally agreed that crowding is the fundamental bottleneck to peripheral object recognition in natural scenes (Levi, 2008; Pelli & Tillman, 2008).
To quantify the resolution of peripheral vision in infants, we have developed the first paradigm to psychophysically measure crowding in infants. Using Mooney faces we examined the ability of 6- to 15-month-old infants to discriminate face orientation (upright or inverted) at three eccentricities in the periphery, in the presence or absence of surrounding flankers. Individual infants’ threshold eccentricities for discriminating the uncrowded and crowded upright face were calculated; this measures the crowding effect, which in turn defines the spatial resolution of visual awareness.
EXPERIMENT 1
Method
Participants
One hundred sixty six healthy, full-term infants participated in the study. They included thirty-seven 6-month-olds (mean age: 6 months 15 days; 20 males and 17 females), forty-six 9-month-olds (mean age: 9 months 14 days; 33 males and 13 females), forty-one 12-month-olds (mean age: 12 months 8 days; 16 males and 25 females), and forty-two 15-month-olds (mean age: 15 months 20 days; 22 males and 20 females). An additional 11 infants were tested but excluded from the final analysis because of failure to complete a minimum of twenty trials (five), fussiness (three), or apparatus malfunction (three). Infants were recruited through letters to parents in Davis, California. The Institutional Review Board at the University of California, Davis, approved the experimental protocol, and informed consent was obtained from a parent or caregiver of each infant.
Stimuli and apparatus
Stimuli consisted of ten Mooney faces, five of which were from the original Mooney study (Mooney, 1957) (Figure 2). Mooney faces lack individual facial features and they cannot be perceived using bottom-up processes, such as parsing or segmenting. Since no cues exist to distinguish the cast shadows in a Mooney face, to find any facial feature, such as an eye or a nose, one must first holistically perceive the image as a face (Cavanagh, 1991; Kemelmacher-Shlizerman, Basri, & Nadler, 2008; Moore & Cavanagh, 1998). In adults, Mooney faces are more difficult to recognize than grayscale photographs of faces, are more easily and rapidly identified as a face when they are in an upright orientation, and activate known face-selective regions such as the fusiform face area (FFA) (Andrews & Schluppeck, 2004; George, Jemel, Fiori, Chaby, & Renault, 2005; Latinus & Taylor, 2005). Recent studies have reported that infants look longer at upright relative to inverted Mooney faces (Doi, Koga, & Shinohara, 2009; Leo & Simion, 2009).
Figure 2.
(a) Mooney face stimuli used in the experiment (top row includes original faces used in Mooney (1957)), (b) upright (left) and inverted (right) without flankers at 3 deg, (c) upright (left) and inverted (right) with flankers at 3 deg. Each face was cropped to fit a 3 deg by 5 deg region; crowded faces were surrounded by six 1.53 deg by 1.05 deg parts (flankers) created from the upright target face, randomly positioned. Flankers were presented with a fixed horizontal center-to-center spacing of 2.2 deg between the face and the flanker. Stimuli were presented against a gray background.
Faces were 99.77% Michelson contrast, and were cropped to fit into a 3 deg by 5 deg ellipse when viewed from a distance of 60 cm. Six 1.05 deg by 1.53 deg flankers were created by “cutting” elliptically-shaped sections from each upright target face. In the crowded condition, flankers were presented surrounding the target faces at a fixed horizontal center-to-center distance of 2.2 deg. Stimuli were presented against a gray background (77.23 cd/m2). Figure 2 illustrates an example trial of uncrowded and crowded faces presented at 3 deg.
The experiment was conducted in a testing room with the lights off. Stimuli were presented on a Tobii 17-inch LCD binocular eye-tracker monitor (1024 × 768 pixels resolution, 50 Hz capture rate, 60 Hz refresh rate). The experiment was programmed and presented using Presentation version 11.3 (Neurobehavioral Systems, Inc.).
Procedure
The experiment began with a five-point calibration routine on the eye-tracker in order to accurately estimate the infant’s gaze point during the task. A video containing a 1 deg dynamic colored image paired with a synchronous sound was presented at the center of the screen until the infant’s fixation was obtained, at which point the experimenter began the trial. A radius of 2 deg around the central fixation point was used as the criteria for central fixation. A trial in which fixation was not obtained within 10 seconds, or the infant’s gaze shifted outside of this radius, was discarded (an average of 7 trials per participant). One upright and one inverted Mooney face (the same face) were shown, each face to the left or right of fixation at a center-to-center distance of 3, 6, or 10 deg along the horizontal meridian. Both faces were presented either with (crowded) or without (uncrowded) corresponding flanker parts for a duration of 2 seconds. The eccentricity at which the faces were presented was blocked in sets of 4 trials and the order of blocks was randomized for each infant. The visual field in which the upright face appeared and the presence of flankers were randomized on each trial. Testing was terminated if the infant did not meet fixation criteria for 5 consecutive trials or became fussy.
Coding and threshold estimation
Eye-tracking data was coded offline using Noldus Observer 5.0 software. The primary measure of performance on each trial was infants’ first saccadic eye movement from the central fixation video immediately following the onset of the faces. First saccades were coded as a hit (1) if fixation landed on the upright face and a miss (0) if fixation landed on the inverted face. In order to make a first saccade to the upright face the infant must have perceived and discriminated the upright face in their visual periphery. Performance correct was thereby calculated as proportion of first saccades to the upright face. Trials where the infant’s fixation remained at the center or where the infant made a saccade to an area on the screen not containing a face were given a score of 0.5 because we assumed this did not indicate discrimination between the stimuli.
To confirm that crowding did not occur when the flanked faces were viewed foveally, we separately calculated an upright face preference score, indexing the proportion of time spent foveating the upright face. This was calculated by dividing the time spent fixating the upright face by the total time spent fixating both faces, ranging from 0 (never looking at upright face) to 1 (only looking at upright face), with 0.5 considered the chance level. The average preference score across the three face locations was used as a measure of foveal (0 deg), or free-viewing, performance because the infants’ gaze was directly on the face and therefore the image must have been foveal.
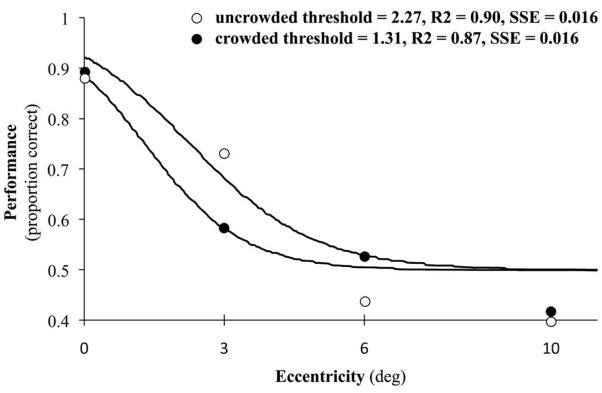
A logistic curve was fit to each infant’s average performance as a function of eccentricity using the psignifit toolbox for Matlab, which implements the maximum-likelihood procedure described by Wichmann and Hill (2001) (Figure 3). Threshold performance was defined as the eccentricity value yielding a performance level of 0.75. To estimate parameters, threshold, slope, and error, a bootstrapping technique was used which included 5000 replications for each fitted function. The criterion for including infants in the analyses was the goodness of fit of the fitted function, evaluated using deviance (Wichmann & Hill, 2001). Individual infant face discrimination thresholds were then used to calculate age group averages.
Figure 3.
Example psychometric functions from a 12-month-old infant. The eccentricity (in deg) at which a performance score of 0.75 was obtained was used as a measure of the threshold for face orientation discrimination, and individual threshold values were used to compare group performance in the uncrowded and crowded conditions.
Results and discussion
A 4 (eccentricity: 0, 3, 6, 10 deg) by 2 (uncrowded or crowded) by 4 (age group: 6, 9, 12, 15 months) repeated measures analysis of variance was conducted on performance (upright face preference score at 0 deg and first saccade score at 3, 6, and 10 deg), which yielded a significant main effect of eccentricity (F(3, 160) = 125.9, p = 0.0001, η2 = 0.703) and crowding (F(1, 162) = 101.1, p = 0.0001, η2 = 0.384), and a significant interaction between eccentricity and crowding (F(3, 160) = 3.823, p = 0.011, η2 = 0.067). There was no main effect of age group (F(3, 162) = 0.51, p = 0.676, η2 = 0.009), and therefore performance was collapsed across all infants for the subsequent analyses. All p-values reported are Bonferroni corrected for multiple comparisons. To examine the interaction, a set of planned comparisons using pair-wise t-tests (2-tailed) was performed which revealed significantly higher performance in the uncrowded (M = 0.63, SD = 0.15) than the crowded (M = 0.59, SD = 0.18) condition only when faces were presented at 3 deg eccentricity (t(165) = 2.336, p = 0.021, SEM = 0.018). At 6 and 10 deg, infants performed no different from chance in either the uncrowded or crowded conditions. To test the magnitude of crowding at 3 degrees for individual infants, a difference score was calculated for each infant by subtracting first saccade performance on crowded trials from first saccade performance on uncrowded trials. Overall, difference scores were significantly positive (t(165) = 2.350, p = 0.020, SEM = 0.018), confirming that the flankers did impact peripheral discrimination at 3 degrees. These results demonstrate that infants’ ability to discriminate the orientation of a Mooney face in the periphery decreased as a function of eccentricity, and was significantly worse in the crowded condition. Critically, flankers did not impair discrimination of the upright face when viewed foveally (t(165) = −1.379, p = 0.170, SEM = 0.014), which is consistent with the definition of crowding and distinguishes it from a masking process that would prevent both detection and identification independent of eccentricity (Pelli, et al., 2004).
Given that there was a significant effect of eccentricity on performance, we calculated individual infants’ face discrimination thresholds separately for the uncrowded and crowded conditions. The final sample of infants whose fitted functions met the goodness of fit criterion included nineteen 6-month-olds, twenty-one 9-month-olds, sixteen 12-month-olds, and twenty-one 15-month-olds. Representative psychometric functions from a 12-month-old infant are shown in Figure 3.
Box plot analyses revealed a skewed distribution of threshold values in all age groups. Because of this non-normality, a trimmed mean threshold was calculated by removing the lowest and highest 20% of the distribution for each age group. Comparing groups based on trimmed means is advantageous for skewed distributions (often found with infant data) because it provides a robust estimate of the most common observation and reduces the effects of extreme values in a sample (Wilcox, 2005).
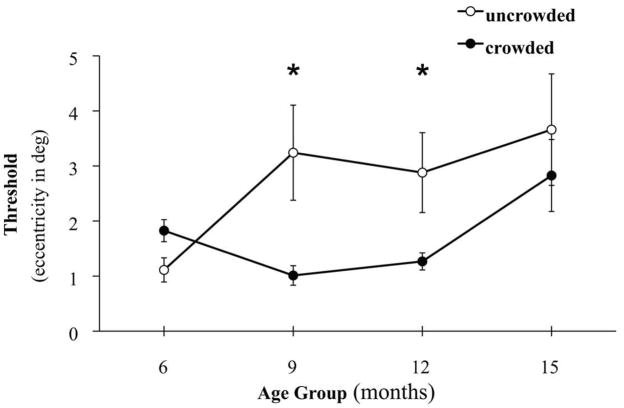
To assess individual infant eccentricity thresholds with age, a one-way analysis of variance using uncrowded or crowded thresholds as the dependent variable and age group (6, 9, 12, or 15 months) as a between-subjects factor revealed a significant main effect of age on crowded thresholds (F(3, 66) = 2.749, p = 0.050, η2 = 0.439), reflecting higher eccentricity limits in 15-month-old infants (Figure 4). Pair-wise t-tests (2-tailed) showed significantly higher thresholds (lower eccentricity limits) in the crowded compared to uncrowded condition for 9-month-old (t(16) = 2.622, p = 0.016, SEM = 0.093) and 12-month-old (t(13) = 2.335, p = 0.029, SEM = 0.189) infants, only.
Figure 4.
Mean uncrowded and crowded thresholds for orientation discrimination of a Mooney face by age group. Asterisks indicate significant differences between pair-wise comparisons (p < 0.05). Error bars represent ± SEM.
These results demonstrate that infants can discriminate the orientation of a Mooney face to a limited extent in the periphery. Critically, the significant interaction between eccentricity and crowding establishes that flankers interfered with discrimination in the periphery, consistent with the definition of crowding. The effect of crowding was observed as close as 3 degrees from fixation across infants of all ages, and could not be evaluated at further eccentricities because performance dropped to chance levels. Threshold values for discrimination of uncrowded faces were unchanged with age, while thresholds for discrimination of crowded faces were significantly lower in 15-month-old infants, showing that the resolution of conscious visual perception increased from 6 to 15 months. However, as a result of the stronger improvement in crowded thresholds, the relative difference in threshold performance for uncrowded and crowded faces was not statistically significant in 15-month-olds (though it trended in the expected direction across age groups), possibly due to a lack of statistical power. As would be expected, a consequence of the developmental change in resolution observed in 15-month-olds is increased variability in performance as a group. In sum, by 15 months of age the spatial resolution of vision was found to be approximately twice as coarse as the resolution measured with the same stimuli in adults (Farzin et al, 2009).
EXPERIMENT 2
In Experiment 1, infants’ abilities to discriminate the orientation of the uncrowded face decreased with eccentricity, most likely as a result of crowding between features within the face, as found in studies of face crowding with adults (Farzin, et al., 2009; Martelli, Majaj, & Pelli, 2005). It could also be argued, however, that the drop in performance is partially the result of infants’ reduced visibility of the faces in the periphery. Experiment 2 inquired whether discrimination of an uncrowded Mooney face in the periphery could be improved by increasing the size of the face, thereby ruling out acuity as the primary limit on conscious vision, and whether, with restored peripheral discrimination performance, the crowding effect would still be present.
Method
Participants
Eleven healthy, full-term infants participated in the study (mean age: 6 months 17 days; 5 males). Infants were recruited through fliers, letters to parents, and word of mouth in Davis, California. The Institutional Review Board at the University of California, Davis, approved the experimental protocol, and informed consent was obtained from a parent or caregiver of each infant.
Stimuli and apparatus
Stimuli were the same faces used in Experiment 1, except that face size was proportionally scaled by a factor of three to fit a 10 deg by 15 deg ellipse when viewed from a distance of 60 cm. Flankers were also scaled by a factor of three such that they fit a 3 deg by 4.75 deg ellipse. To remove overlap between central fixation and the most foveal flankers and to ensure that the most peripheral flankers were visible on the screen, the crowded condition consisted of four flankers rather than six, presented surrounding the faces at a fixed horizontal center-to-center distance of 7 deg.
The apparatus was identical to that described in Experiment 1.
Procedure
The procedure of Experiment 2 was identical to that of Experiment 1 except that one upright and one inverted face were shown at ± 10 deg from fixation, along the horizontal meridian.
Coding
As in Experiment 1, infants’ first saccadic eye movement from the central fixation was coded and discrimination performance was calculated as proportion of first saccades made to the upright face.
Results and discussion
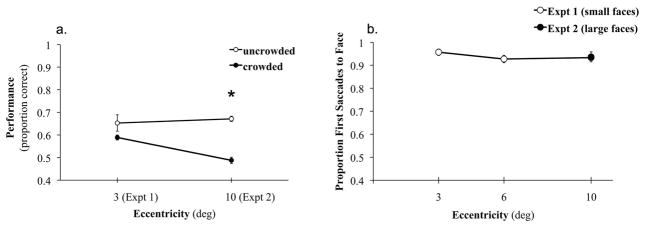
Infants’ orientation discrimination performance for uncrowded faces presented at 10 deg was significantly better with increased face size, as compared to performance obtained with smaller faces at 10 deg in Experiment 1 (F(1, 176) = 42.98, p = 0.0001, η2 = 0.197). Further, performance with the larger uncrowded faces at 10 deg was equivalent to performance observed in Experiment 1 with smaller uncrowded faces at 3 deg (Figure 5a). The effect of flankers was tested using a pair-wise t-test (2-tailed), which revealed a significant difference between performance in the uncrowded and crowded conditions (t(10) = 5.128, p = 0.0001, SEM = 0.036) such that discrimination of larger faces in the presence of flankers did not differ from the chance level of 0.5 (t(10) = −1.096, p = 0.299, SEM = 0.011). Together, these data illustrate that increasing face size restored discrimination performance in the periphery, and that when the visibility of faces at 3 and 10 deg was equated, flankers continued to impair recognition of the upright face. This confirms that beyond limitations in peripheral acuity, there is a coarser limit to peripheral object recognition imposed by crowding.
Figure 5.
(a) Infants’ orientation discrimination performance (first saccade score) for the larger uncrowded faces at 10 deg was equal to performance observed in Experiment 1 for smaller uncrowded faces at 3 deg, suggesting that infants can distinguish a face at 10 deg. Flankers around the large face at 10 deg reduced discrimination to chance. This confirms that discrimination in the periphery is limited most fundamentally by crowding, not just visibility or acuity. Asterisk indicates significant differences between pair-wise comparisons (p < 0.05). Error bars represent ± SEM. (b) proportion of trials at each eccentricity in which a face (either the upright or inverted) was correctly localized.
An additional analysis was conducted to confirm that infant peripheral detection capabilities could not account for the drop in performance with eccentricity. The proportion of trials from Experiments 1 and 2 in which the infant’s first saccade landed on one of the face images (either upright or inverted) was calculated for the uncrowded condition as a function of eccentricity. If infants were unable to detect the faces at more peripheral locations due to limitations in acuity, first saccade localization accuracy would be expected to decline with eccentricity. This analysis indicated that infants were, in fact, able to detect and localize the spatial position of the faces very precisely across all eccentricities, irrespective of face size. Figure 5b shows the proportion of trials in which one of the faces was correctly localized at each eccentricity. Thus, similar to adults, acuity is not the main limiting factor on peripheral visual awareness in infants—crowding is.
General discussion
Crowding limits our conscious visual perception of individual objects in naturally cluttered scenes, thereby defining the window of object recognition in the periphery. The present study measured the effective spatial resolution of peripheral visual perception in infants. Experiment 1 established that infants between the ages of 6 and 15 months can recognize a Mooney face in the periphery and the results demonstrated that the presence of surrounding flankers significantly reduced this ability as a function of increasing eccentricity. Infants’ face discrimination thresholds revealed a spatial resolution of conscious perception twice as coarse as that of adults, substantially limiting what an infant can perceive in a peripheral scene. Unlike adults, who employ a fine-grained visual “spotlight” to access peripheral information, infants appear to have a more diffuse “lantern” of visual awareness that sets the limit on what can be registered and accessed in the periphery. Thus, an infant’s visual experience may not simply be more blurred than that of adults, as would be predicted by studies showing immature acuity and contrast sensitivity, rather their visual world may include more of a jumbled mass of unbound features relative to what adults experience.
These results confirm that infants’ inability to recognize a face in the presence of flankers with increasing eccentricity was the result of crowding and cannot be attributed to reduced visibility or some other phenomenon. The pair of Mooney faces differed only in orientation, ensuring that discrimination was not based on low-level visual cues such as differences in internal elements or mean luminance. The primary measure—infants’ first saccadic eye movement from central fixation to one of the faces—ensured that perception of the face relied on peripheral vision and that the flankers did not simply distract the infant away from the face. Infants’ discrimination of the uncrowded upright face in the periphery was restored when face size was increased; in the presence of flankers, however, the face was still crowded and unrecognizable. Together, these results demonstrate the first quantitative measure of crowding in infants.
The finding that young infants experience a much coarser resolution of conscious visual perception compared to adults has implications for our understanding of visual and visuomotor development. First, it illustrates that the limit of peripheral awareness is experience-dependent. Regardless of the neural mechanism(s) used to explain crowding, this fundamental limit on object recognition is the result of a developmental process. That is, the size of the “spotlight”, or window, which defines the spatial resolution of visual awareness, is flexible and may shrink across a certain developmental period, improving peripheral recognition of cluttered scenes over time until adult levels are reached. This has further implications for visually-guided actions. Because many eye and reaching movements require peripheral recognition of individual objects, limited spatial resolution may also influence or constrain the development of goal-directed actions that can be executed to the periphery (Bulakowski, Post, & Whitney, 2009). Indeed, the limited spatial resolution of peripheral perception may contribute to age-related changes in the frequency of eye movements during infancy (Salapatek, Aslin, Simonson, & Pulos, 1980).
An intriguing question that arises from this study is whether infants’ limited awareness represents a tradeoff between spatial coverage and resolution, and, if so, whether reduced resolution may actually be advantageous for infants. For example, some evidence suggests that, while crowding blocks access to individual identities in the periphery, access to global or gist statistics is preserved (Haberman & Whitney, 2007; Larson & Loschky, 2009; Parkes, Lund, Angelucci, Solomon, & Morgan, 2001). Therefore, limited conscious vision may serve a useful purpose for young infants by allowing extraction of information about the gist of a scene in a computationally efficient and unencumbered way. Insofar as typical neural and cognitive maturation depend on perception and attention, future research on infant development needs to consider the realistic limitation imposed by crowding in natural scenes—the spatial resolution of conscious vision.
Acknowledgments
The authors thank the infants and their parents for participating, Patrick Cavanagh for providing many of the Mooney faces, and David Horton, Jason Fischer, and Paul Bulakowski for valuable comments on earlier drafts. Parts of these data were presented at the Vision Sciences Society Annual Meeting in 2008. This work was supported by NIH grants F31MH083386 (F.F.) and EY018216 (D.W.), and NSF grant 0748689 (D.W.).
Contributor Information
Faraz Farzin, Department of Psychology, University of California, Davis, Center for Mind and Brain, Davis, California.
Susan M. Rivera, Department of Psychology, University of California, Davis, Center for Mind and Brain, Davis, California, M.I.N.D. Institute, Sacramento, California
David Whitney, Department of Psychology, University of California, Berkeley, Center for Mind and Brain, Davis, California.
References
- Andrews TJ, Schluppeck D. Neural responses to Mooney images reveal a modular representation of faces in human visual cortex. NeuroImage. 2004;21:91–98. doi: 10.1016/j.neuroimage.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Andriessen JJ, Bouma H. Eccentric vision: Adverse interactions between line segments. Vision Research. 1976;16:71–78. doi: 10.1016/0042-6989(76)90078-x. [DOI] [PubMed] [Google Scholar]
- Bouma H. Interaction effects in parafoveal letter recognition. Nature. 1970;226:177–178. doi: 10.1038/226177a0. [DOI] [PubMed] [Google Scholar]
- Bulakowski PF, Post RB, Whitney D. Visuomotor crowding: the resolution of grasping in cluttered scenes. Frontiers in Behavioral Neuroscience. 2009;3(49):1–7. doi: 10.3389/neuro.08.049.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh P. What’s up in top-down processing? In: Gorea A, editor. Representations of Vision: Trends and tacit assumptions in vision research. 1991. pp. 295–304. [Google Scholar]
- Cohen LB. Attention-getting and attention-holding processes of infant visual preferences. Child Development. 1972;43(3):869–879. [PubMed] [Google Scholar]
- de Schonen S, McKenzie B, Maury L, Bresson F. Central and peripheral object distances as determinants of the effective visual field in early infancy. Perception. 1978;7:499–506. doi: 10.1068/p070499. [DOI] [PubMed] [Google Scholar]
- Doi H, Koga T, Shinohara K. 18-Month-olds can perceive Mooney faces. Neuroscience Research. 2009;64(3):317–322. doi: 10.1016/j.neures.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Farzin F, Rivera SM, Whitney D. Holistic crowding of Mooney faces. Journal of Vision. 2009;9(6):1–15. doi: 10.1167/9.6.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flom MC, Heath GG, Takahashi E. Contour interaction and visual resolution: Contralateral effect. Science. 1963;142:979–980. doi: 10.1126/science.142.3594.979. [DOI] [PubMed] [Google Scholar]
- George N, Jemel B, Fiori N, Chaby L, Renault B. Electrophysiological correlates of facial decisions: Insights from upright and upside-down Mooney-face perception. Cognitive Brain Research. 2005;24(3):663–673. doi: 10.1016/j.cogbrainres.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Gopnik A. The philosphical baby: what children’s minds tell us about truth, love, and the meaning of life. New York: Farrar, Straus, and Giroux; 2009. [Google Scholar]
- Haberman J, Whitney D. Rapid extraction of mean emotion and gender from sets of faces. Current Biology. 2007;17(17):751–753. doi: 10.1016/j.cub.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Cavanagh P, Intriligator J. Attentional resolution. Trends in Cognitive Sciences. 1997;1(3):115–121. doi: 10.1016/S1364-6613(97)89058-4. [DOI] [PubMed] [Google Scholar]
- Intriligator J, Cavanagh P. The spatial resolution of visual attention. Cognitive Psychology. 2001;43:171–216. doi: 10.1006/cogp.2001.0755. [DOI] [PubMed] [Google Scholar]
- Kemelmacher-Shlizerman I, Basri R, Nadler B. 3D shape reconstruction of Mooney faces. Paper presented at the IEEE Conference on Computer Vision and Pattern Recognition.2008. [Google Scholar]
- Kooi FL, Toet A, Tripathy SP, Levi DM. The effect of similarity and duration on spatial interaction in peripheral vision. Spatial Vision. 1994;9:255–279. doi: 10.1163/156856894x00350. [DOI] [PubMed] [Google Scholar]
- Larson AM, Loschky LC. The contributions of central versus peripheral vision to scene gist recognition. Journal of Vision. 2009;9(10):1–16. doi: 10.1167/9.10.6. [DOI] [PubMed] [Google Scholar]
- Latinus M, Taylor MJ. Holistic processing of faces; learning effects with Mooney faces. Journal of Cognitive Neuroscience. 2005;17:1316–1327. doi: 10.1162/0898929055002490. [DOI] [PubMed] [Google Scholar]
- Leo I, Simion F. Newborns’ Mooney-face perception. Infancy. 2009;14(6):641–653. doi: 10.1080/15250000903264047. [DOI] [PubMed] [Google Scholar]
- Levi DM. Crowding-An essential bottleneck for object recognition: A mini-review. Vision Research. 2008;48(5):635–654. doi: 10.1016/j.visres.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie EG, Bressler DW, Whitney D. Holistic crowding: selective interference between configural representations of faces in crowded scenes. Journal of Vision. 2007 doi: 10.1167/7.2.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P, Näsänen R, Rovamo J, Melmoth D. Identification of facial images in peripheral vision. Vision Research. 2001;41:599–610. doi: 10.1016/s0042-6989(00)00259-5. [DOI] [PubMed] [Google Scholar]
- Martelli M, Majaj NJ, Pelli DG. Are faces processed like words? A diagnostic test for recognition by parts. Journal of Vision. 2005;5:58–70. doi: 10.1167/5.1.6. [DOI] [PubMed] [Google Scholar]
- Maurer D, Lewis T. Peripheral discrimination by three-month-old infants. Child Development. 1979;50:276–279. [PubMed] [Google Scholar]
- McKone E. Isolating the special component of face recognition: peripheral identification and a Mooney face. Journal of Experimental Psychology Learning Memory and Cognition. 2004;30(1):181–197. doi: 10.1037/0278-7393.30.1.181. [DOI] [PubMed] [Google Scholar]
- McKone E, Kanwisher N, Duchaine BC. Can generic expertise explain special processing for faces? Trends in Cognitive Sciences. 2007;11(1):8–15. doi: 10.1016/j.tics.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Mooney CM. Age in the development of closure ability in children. Canadian Journal of Psychology. 1957;11:216–226. doi: 10.1037/h0083717. [DOI] [PubMed] [Google Scholar]
- Moore C, Cavanagh P. Recovery of 3D volume from 2-tone images of novel objects. Cognition. 1998;67:45–71. doi: 10.1016/s0010-0277(98)00014-6. [DOI] [PubMed] [Google Scholar]
- Parkes L, Lund J, Angelucci A, Solomon JA, Morgan M. Compulsory averaging of crowded orientation signals in human vision. Nature Neuroscience. 2001;4(7):739–744. doi: 10.1038/89532. [DOI] [PubMed] [Google Scholar]
- Pelli DG, Palomares M, Majaj NJ. Crowding is unlike ordinary masking: distinguishing feature integration from detection. Journal of Vision. 2004;4:1136–1139. doi: 10.1167/4.12.12. [DOI] [PubMed] [Google Scholar]
- Pelli DG, Tillman KA. The uncrowded window of object recognition. Nature Neuroscience. 2008;11(10):1120–1135. doi: 10.1038/nn.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salapatek P. Pattern perception in early infancy. In: Cohen LB, Salapatek P, editors. Infant Perception. New York: Academic Press; 1975. [Google Scholar]
- Salapatek P, Aslin RN, Simonson J, Pulos E. Infant saccadic eye movements to visible and previously visible targets. Child Development. 1980;51:1090–1094. [PubMed] [Google Scholar]
- Sireteanu R, Fronius M, Constantinescu DH. The development of visual acuity in the peripheral visual field of human infants: binocular and monocular measurements. Vision Research. 1994;34(12):1659–1671. doi: 10.1016/0042-6989(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Strasburger H, Harvey LO, Rentschler I. Contrast thresholds for identification of numeric characters in direct and eccentric view. Perception & Psychophysics. 1991;49(6):495–508. doi: 10.3758/bf03212183. [DOI] [PubMed] [Google Scholar]
- Townsend JT, Taylor SG, Brown DR. Lateral masking for letters with unlimited viewing time. Perception and Psychophysics. 1971;10:375–378. [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: II. Bootstrap-based confidence intervals and sampling. Perception and Psychophysics. 2001;63:1314–1329. doi: 10.3758/bf03194545. [DOI] [PubMed] [Google Scholar]
- Wilcox RR. Introduction to robust estimation and hypothesis testing. 2. Burlington: Elsevier; 2005. [Google Scholar]