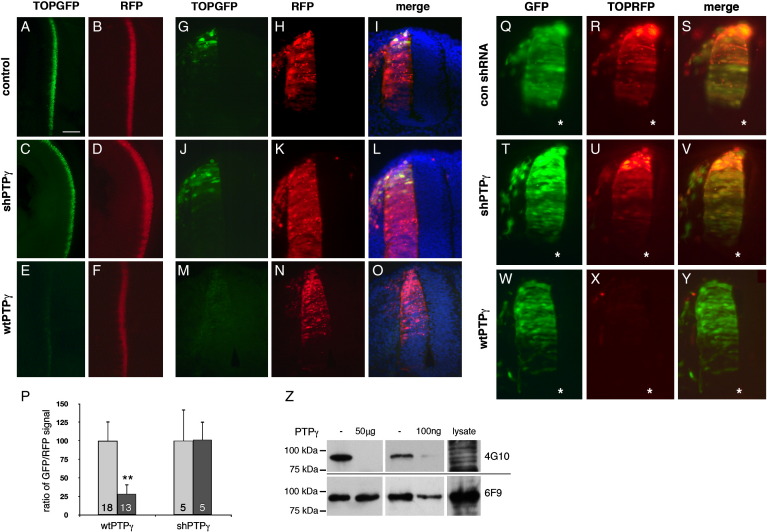
Fig. 6.
PTPγ gain-of-function suppresses TCF-dependent transcription. To detect TCF activity, embryos were electroporated with TOPGFP along with an electroporation reporter (RFP) and either a control shRNA plasmid (A, B, and G–I), the Si3 vector (shPTPγ; C, D, and J–L) or wtPTPγ expression vector (E, F, and M–O). A–F, wholemount images of embryo trunks viewed dorsally. G–O are sections of similar embryos (dorsal top), showing GFP expression, RFP expression and image merges with DAPI. A sharp, dorsoventral gradient of TOPGFP is visible (G and J). Total GFP and RFP signals were quantified and GFP/RFP ratios determined and graphed (P) (see Experimental methods). In P, each column contains the sample size; error bars represent SD (* P < 0.01; ** P < 0.001). Q–Y show similar assays with a more sensitive TOPRFP reporter (and GFP electroporation reporter), in HH22 spinal cords. TOPRFP reveals a full dorsoventral gradient of TCF activity, which is similar in control shRNA- and Si3-treated embryos (R and U, respectively). X shows almost complete extinction of TOPRFP signal after wtPTPγ expression. Z shows in vitro dephosphorylation of β-catenin protein by PTPγ. β-catenin was immunopurified from 293 T cells and incubated with either 50 μg or 100 ng purified human PTPγ D1/D2 catalytic domains. Samples were immunoblotted to detect phosphotyrosine and β-catenin. Whole cell lysate samples are also shown. Scale bar = 500 μm (A–F) 50 μm (G–Y).