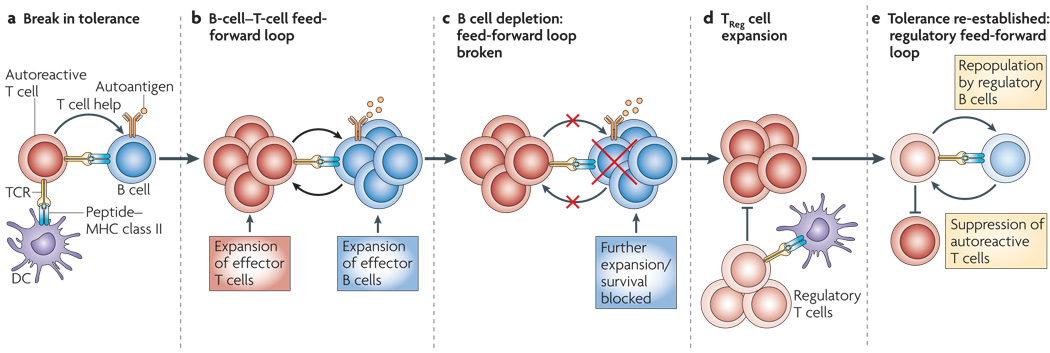
Figure 1. Resetting the effector and regulatory B and T cell networks after B cell depletion.
The mechanistic basis for the effectiveness of B cell depletion in treating autoimmune disease is not understood. Based on the data, we propose a model in which autoreactive effector B and T cells establish a feed-forward loop that destroys the interconnected effector and regulatory B and T cell networks. B cell depletion allows for re-establishment of the regulatory network and favors development of tolerance upon B cell repopulation. In this model, a break in tolerance of either the B or T cell occurs (panel a), allowing for the initiation of cognate interactions between autoreactive T and B cells and the establishment of a B and T cell dependent feed-forward loop (panel b). This antigen-driven feed-forward loop eventually dominates the response and is no longer susceptible to suppression by regulatory B and T cells. When B cells are depleted (panel c), the T/B feed-forward loop is broken and the number of responding T cells decreases. This results in decreased inflammatory cytokine production by the autoreactive T and B cells favors the generation, expansion and function of regulatory T cells (panel d) that can suppress remaining autoreactive effector T cells. Finally, the transitional and naïve B cells that initially repopulate the patient (panel e) are likely to have regulatory or tolerance inducing capacity, similar to the B cells found in neonates. These B cells can drive the induction or expansion of new cohorts of regulatory T cells, resetting the balance of regulatory and effector T cells to favor tolerance rather than responsiveness.