Abstract
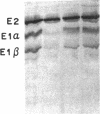
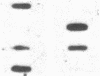
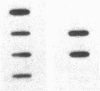
Maple syrup urine disease (MSUD) results from a deficiency of branched chain alpha-ketoacid dehydrogenase (BCKDH). We have studied the etiology of MSUD by determining the enzyme activity, protein, and mRNA levels of BCKDH in fibroblasts from a classic MSUD patient and his parents. By enzymatic amplification of the patient's mRNA followed by cloning and DNA sequencing, we have identified a T to A transversion that alters a tyrosine to an asparagine at residue 394 of the E1 alpha subunit. Amplification of both mRNA and genomic DNA, in combination with allele-specific oligonucleotide hybridization, demonstrated that the father was heterozygous for this mutant allele. The mother was homozygous for the allele encoding the normal Tyr394, but expressed only about half of the normal level of mRNA and protein. The patient was genetically heterozygous for this altered allele, although only the abnormal allele was expressed as mRNA. We conclude that the patient was a compound heterozygote, inheriting an allele encoding an abnormal E1 alpha from the father, and an allele from the mother containing a cis-acting defect in regulation which abolished the expression of one of the E1 alpha alleles. Our results revealed for the first time that a case of MSUD was caused by structural and regulatory mutations involving the E1 alpha subunit.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chuang D. T., Cox R. P. Enzyme assays with mutant cell lines of maple syrup urine disease. Methods Enzymol. 1988;166:135–146. doi: 10.1016/s0076-6879(88)66020-4. [DOI] [PubMed] [Google Scholar]
- Chuang D. T., Ku L. S., Kerr D. S., Cox R. P. Detection of heterozygotes in maple-syrup-urine disease: measurements of branched-chain alpha-ketoacid dehydrogenase and its components in cell cultures. Am J Hum Genet. 1982 May;34(3):416–424. [PMC free article] [PubMed] [Google Scholar]
- Damuni Z., Merryfield M. L., Humphreys J. S., Reed L. J. Purification and properties of branched-chain alpha-keto acid dehydrogenase phosphatase from bovine kidney. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4335–4338. doi: 10.1073/pnas.81.14.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner D. J., Armstrong N., Heffelfinger S. C., Sewell E. T., Priest J. H., Elsas L. J. Absence of branched chain acyl-transferase as a cause of maple syrup urine disease. J Clin Invest. 1985 Mar;75(3):858–860. doi: 10.1172/JCI111783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr C. J., Saiki R. K., Erlich H. A., McCormick F., Marshall C. J. Analysis of RAS gene mutations in acute myeloid leukemia by polymerase chain reaction and oligonucleotide probes. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1629–1633. doi: 10.1073/pnas.85.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatania H. R., Lau K. S., Randle P. J. Inactivation of purified ox kidney branched-chain 2-oxoacid dehydrogenase complex by phosphorylation. FEBS Lett. 1981 Sep 28;132(2):285–288. doi: 10.1016/0014-5793(81)81180-5. [DOI] [PubMed] [Google Scholar]
- Indo Y., Kitano A., Endo F., Akaboshi I., Matsuda I. Altered kinetic properties of the branched-chain alpha-keto acid dehydrogenase complex due to mutation of the beta-subunit of the branched-chain alpha-keto acid decarboxylase (E1) component in lymphoblastoid cells derived from patients with maple syrup urine disease. J Clin Invest. 1987 Jul;80(1):63–70. doi: 10.1172/JCI113064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mackall J., Meredith M., Lane M. D. A mild procedure for the rapid release of cytoplasmic enzymes from cultured animal cells. Anal Biochem. 1979 May;95(1):270–274. doi: 10.1016/0003-2697(79)90216-1. [DOI] [PubMed] [Google Scholar]
- Paxton R., Harris R. A. Isolation of rabbit liver branched chain alpha-ketoacid dehydrogenase and regulation by phosphorylation. J Biol Chem. 1982 Dec 10;257(23):14433–14439. [PubMed] [Google Scholar]
- Paxton R., Kuntz M., Harris R. A. Phosphorylation sites and inactivation of branched-chain alpha-ketoacid dehydrogenase isolated from rat heart, bovine kidney, and rabbit liver, kidney, heart, brain, and skeletal muscle. Arch Biochem Biophys. 1986 Jan;244(1):187–201. doi: 10.1016/0003-9861(86)90108-6. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Yeaman S. J., Reed L. J. Purification and characterization of branched chain alpha-keto acid dehydrogenase complex of bovine kidney. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4881–4885. doi: 10.1073/pnas.75.10.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler I. D., Kerr D. S., Ho L., Lusk M. M., Pepin R. A., Javed A. A., Mole J. E., Jesse B. W., Thekkumkara T. J., Pons G. Heterogeneous expression of protein and mRNA in pyruvate dehydrogenase deficiency. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7336–7340. doi: 10.1073/pnas.85.19.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Crabb D. W., Harris R. A. Nucleotide and deduced amino acid sequence of the E1 alpha subunit of human liver branched-chain alpha-ketoacid dehydrogenase. Gene. 1988 Sep 15;69(1):159–164. doi: 10.1016/0378-1119(88)90390-3. [DOI] [PubMed] [Google Scholar]
- Zhang B., Kuntz M. J., Goodwin G. W., Harris R. A., Crabb D. W. Molecular cloning of a cDNA for the E1 alpha subunit of rat liver branched chain alpha-ketoacid dehydrogenase. J Biol Chem. 1987 Nov 5;262(31):15220–15224. [PubMed] [Google Scholar]