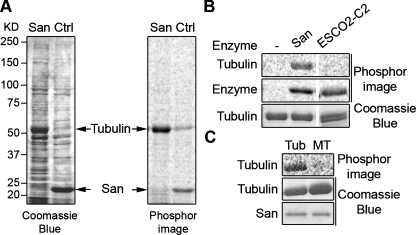
FIGURE 1:
San acetylates tubulin heterodimers but not MTs. (A) Tubulin is a San substrate. HeLa cell lysate was incubated with either San-conjugated NHS beads (San) or empty beads (Ctrl), and proteins bound to the beads were analyzed in the in vitro San acetylation assay. For the empty beads control, recombinant San was added after the pull-down experiment. After resolving the proteins by SDS–PAGE, the gel was stained with Coomassie Blue (left panel) and exposed to phosphorimage (right panel). The 52 kDa protein band was identified as tubulin by MS. In the San beads sample, San proteins were covalently linked to the beads and therefore not visible on the gel. (B) Tubulins were acetylated by San but not ESCO2. Bovine tubulins were subjected to in vitro acetylation with buffer (–), San, or ESCO2-C2 (Hou and Zou, 2005). The tubulin doublet shown in the third lane was due to partial separation of α- and β-tubulin on the gel. The self-acetylations of San and ESCO2-C2 were used as controls for enzymatic activity. (C) Tubulins but not MTs were acetylated by San. MTs made of bovine tubulins were either depolymerized to tubulins by incubation on ice (Tub) or stabilized by Taxol (MT). Both preparations were subjected to in vitro acetylation with San.