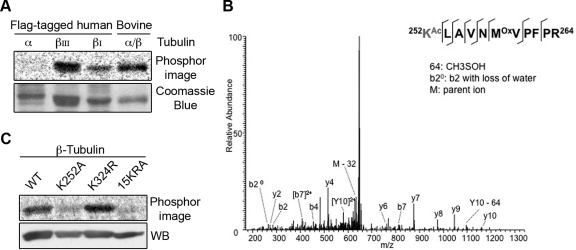
FIGURE 4:
San acetylates β-tubulin at K252. (A) San acetylated β- but not α-tubulin. Flag-tagged tubulins were expressed in 293T cells, purified on anti-flag affinity gel, and analyzed by the in vitro acetylation assay. Bovine tubulin serves as a positive control. (B) Identification of K252 acetylation on βI-tubulin in vitro. MS/MS spectrum of m/z 664.7 identifies K252 as being acetylated. The labels b and y designate the N- and C-terminal fragments, respectively, of the peptide produced by breakage at the peptide bond in the mass spectrometer. The number represents the number of N- or C-terminal residues present in the peptide fragment. KAc and Mox represent acetyl-lysine and oxidized methionine residues, respectively. (C) β-tubulin K252A mutant was not efficiently acetylated by San in vitro. Wild-type and mutant β-tubulins were expressed in 293T cells, purified on anti-flag affinity gel, eluted by TEV protease, and analyzed by the in vitro acetylation assay. Immunoblot with β-tubulin antibody demonstrated equal loading.