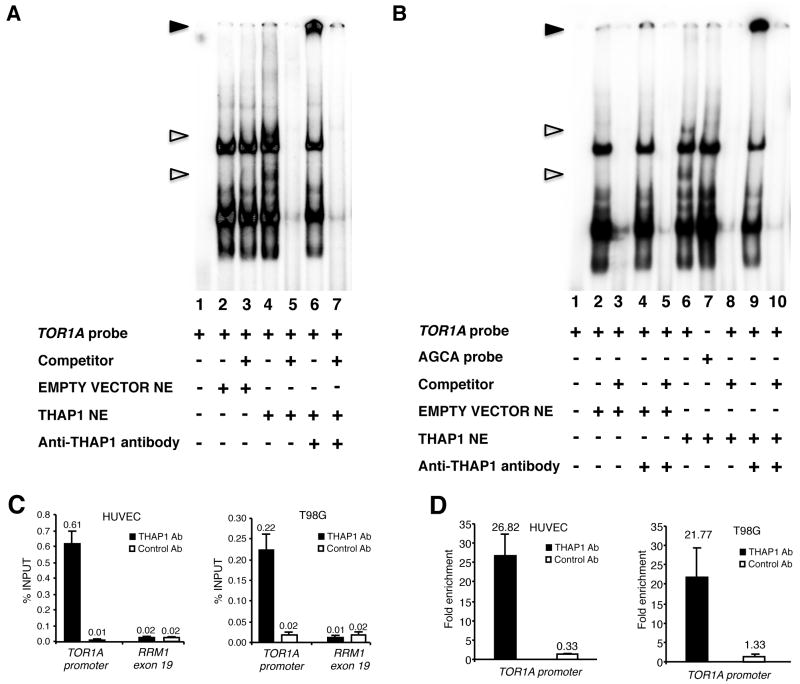
Figure 1.
THAP1 binds the TOR1A promoter in vitro and in vivo. (A,B) Nuclear extracts (NE) from 293T cells transfected with wildtype human THAP1 were subjected to electrophoretic mobility shift assay (EMSA) using the TOR1A promoter region −159/−80 as a probe in A, or the same probe mutated in the THAP1 core consensus site (AGCA probe) in B. Unfilled triangles indicate specific nuclear complexes. Filled triangles indicate the supershifted bands. (C,D) ChIP-qPCR assays were used to analyze association of THAP1 with the TOR1A promoter in vivo. Cross-linked chromatin from HUVEC primary cells or T98G cells was subjected to immunoprecipitation with antibodies against THAP1 (THAP1 Ab1) or negative control antibodies (Control Ab). The human RRM1-exon 19 genomic region was used as a control genomic region. (C) Immunoprecipitated DNA was quantified by qPCR using the percent of input method. (D) Fold enrichment of THAP1 on the TOR1A promoter was calculated by dividing the amount of TOR1A promoter DNA precipitated by anti-THAP1 antibodies to the amount of DNA precipitated from the RRM1-exon 19 genomic region. ChIP results are shown as means with SD from three separate datapoints.