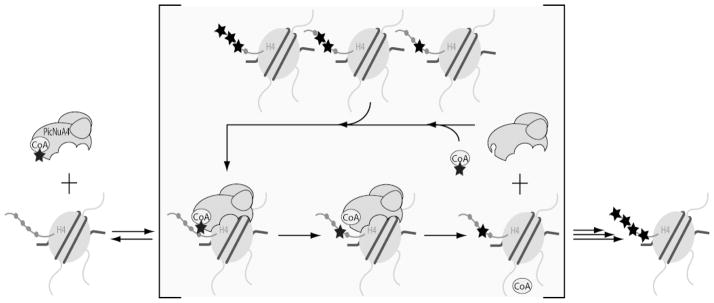
Figure 6. Proposed dissociative model of nucleosome acetylation by picNuA4.
Diagram describes several kinetic steps required for recognition and processing of nucleosomal substrates by picNuA4. After binding AcCoA, picNuA4 binds the H4 histone fold domain while the active site effectively scans the histone tail for lysine residues surrounded by small, uncharged aliphatic residues. Preferred sites of acetylation maintain a minimal (n + 2) register between lysines. Upon catalysis, picNuA4 releases both the acetylated product and CoA before the next cycle of catalysis.