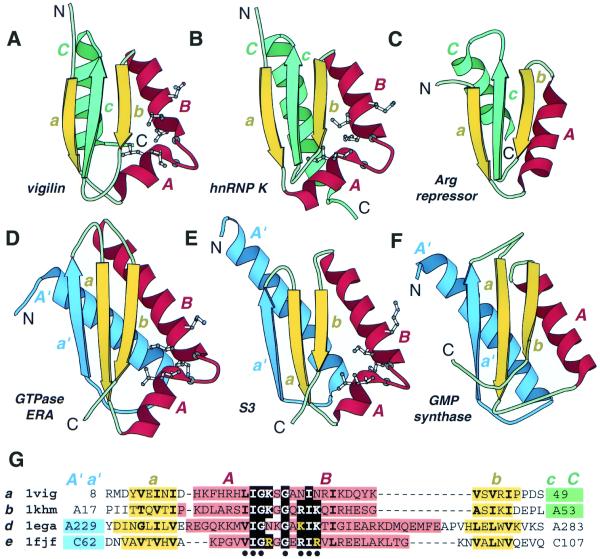
Figure 1.
Structural comparison of KH domains. Ribbon diagrams of type I (maxi) KH domains (A and B), type II (mini) KH domains (D and B) and non-KH proteins (C and F) were drawn by Bobscript (36), a modified version of Molscript (37). The structures were superimposed and then separated for clarity. N- and C-termini are labeled. The spatially equivalent structural elements are colored correspondingly. N- and C-terminal extension in type II (mini) and type I (maxi) KH domains and their structural equivalencies in non-KH proteins of similar fold are colored in blue and green, respectively. α-Helices and β-strands are labeled in upper and lower case italic letters, respectively. Letter color matches the color of the secondary structure element. Side chains (Cα atoms for Gly) of residues conserved in KH domains are displayed. (A) Repeat 6 of vigilin [PDB (29) entry 1VIH, residues 7–76]; (B) C-terminal KH domain of hnRNP K (PDB entry 1KHM, residues A11–A89); (C) C-terminal domain of E.coli arginine repressor (PDB entry 1XXA, residues A92–A152, the first β-strand is not shown); (D) C-terminal domain of GTPase ERA (PDB entry 1EGA, residues A186–A283); (E) N-terminal domain of ribosomal protein S3 (PDB entry 1FJF, residues C24–C106); (F) C-terminal domain of E.coli GMP synthetase (PDB entry 1GPM, residues A416–A523); (G) structure-based sequence alignment of KH motif regions from structures shown in (A, B, D and E). The panel label, PDB entry name, starting and ending residue numbers are given for each protein. Color shading and labels of secondary structure elements correspond to those in A–F. Highly conserved residues in KH domains (KH signature) are boxed with black. Hydrophobic amino acids in buried sites are shown in bold letters. Blue and green shading points to the presence of N- and C-terminal extensions whose sequences are not shown. The residues with side chains (Cα atoms for Gly) displayed on ribbon diagrams are marked with a black dot below the alignment.