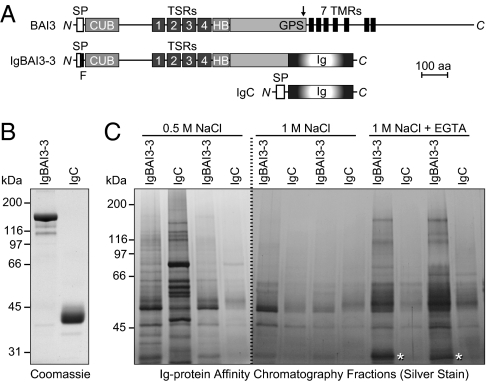
Fig. 1.
Identification of C1ql proteins as BAI3 ligands. (A) Schematic depiction of the domain organization of BAI3 (1,522 amino acids), of the BAI3 Ig-fusion protein IgBAI3-3, and of the control Ig-fusion protein IgC. SP, signal peptide; F, FLAG tag; CUB, complement C1r/C1s-Uegf-Bmp1 domain; TSRs, thrombospondin repeats; HB, hormone-binding domain; GPS, GPCR proteolysis sequence (proteolytic site denoted by an arrow); 7 TMR, seven-transmembrane region; Ig, Fc domain of human IgG. (B) Coomassie blue–stained SDS gel of Ig-fusion proteins produced in HEK293 cells and purified by protein A–Sepharose affinity chromatography. (C) Silver-stained SDS gel of a representative pull-down experiment. Mouse brain homogenates were incubated with protein A–Sepharose beads containing either IgBAI3-3 or IgC. After the beads were washed, bound proteins were sequentially eluted with buffers containing 0.5 M NaCl, 1 M NaCl, and 1 M NaCl with 5 mM EGTA. Two fractions per buffer were collected and analyzed. Mass spectrometry analysis identified C1ql2 and C1ql3 in the band marked by an asterisk.