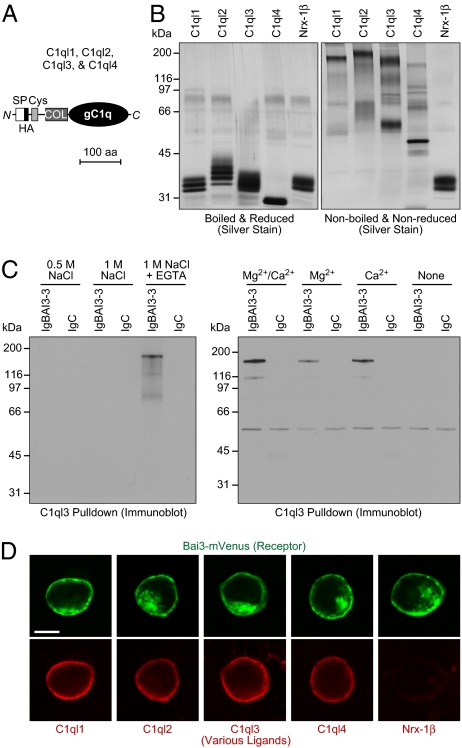
Fig. 2.
Characterization of BAI3 binding by C1ql proteins. (A) Domain structure of C1ql proteins C1ql1–C1ql4 (238–287 amino acids). SP, signal peptide; Cys, cysteine loop domain; COL, collagen-like domain; gC1q, globular C1q domain. An HA epitope was inserted immediately following the signal peptide. (B) Silver-stained SDS gel of purified HA-tagged full-length C1ql proteins and of a recombinant protein containing the extracellular neurexin-1β sequences loaded onto SDS gels with (Left) or without (Right) reducing agents and boiling. Proteins were expressed in HEK293 cells and purified from the medium by anti-HA agarose. (C) Analyses of C1ql3-mediated pull-downs of recombinant IgBAI3-3 protein, using IgC as a negative control. HA-tagged C1ql3 immobilized on an anti-HA column was incubated with IgBAI3-3 or IgC, and bound proteins were analyzed by immunoblot analysis for human Ig. (Left) Eluates obtained after binding in Ca2+/Mg2+-containing buffer and elution with the indicated solutions. (Right) Proteins bound to immobilized C1ql3 in buffers containing the indicated divalent cations. (D) Cell-surface labeling assay demonstrating that all four C1ql isoforms bind to BAI3. HEK293 cells transfected with a vector encoding mVenus-tagged BAI3 (green) were incubated with purified HA-tagged C1ql1–C1ql4 and neurexin-1β (extracellular sequences). Bound proteins were visualized by immunofluorescence labeling (red). (Scale bar: 10 μm.)