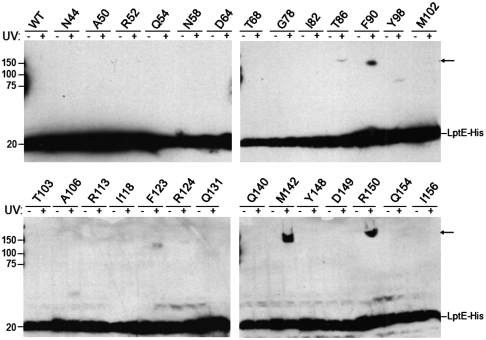
Fig. 1.
In vivo photocrosslinking of LptE. E. coli expressing low levels of wild-type or pBPA-substituted LptE-His protein, as indicated, in a chromosomal ΔlptE background were either left untreated or irradiated with UV light, followed by Western blot analysis of whole-cell lysates with an anti-His antibody. Arrows, UV-photocrosslinked complexes containing pBPA-substituted LptE-His. The analyzed positions were selected from the 193 residues of full-length LptE (residue numbers include the signal sequence, a.a. 1–18) on the basis of structural conservation with LptE orthologs from other Gram-negative bacteria: no structural information exists for residues 19–35, and residues 166–193 of E. coli LptE constitute a C-terminal extension that is not found in other organisms (see Fig. 2B).