Fig. 4.
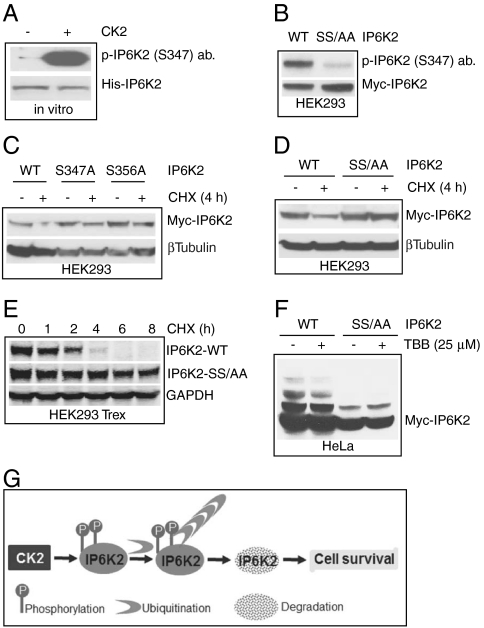
IP6K2 destabilization is associated with CK2 phosphorylation at serines 347 and 356 A. CK2 phosphorylation of bacterially purified His-tagged IP6K2 is detected by α-pIP6K2 (S347) antibody. Total protein was detected by coommassie blue staining. B. Myc-IP6K2 S347/356AA double mutant is not recognized by the α-pIP6K2 (S347) antibody. WT and mutant IP6K2 were overexpressed in HEK293 cells and were immunoprecipitated using α-Myc antibody. C. IP6K2 S347A and S356A single mutants display partial but not total stability after cycloheximide treatment. D. IP6K2 S347/356AA double mutant does not degrade after 4 h cycloheximide treatment. E. Substantial increase in half-life of IP6K2 S347/356AA double mutant. The half-life of the mutant is 8 h as compared to 2 h for WT IP6K2. F.TBB decreases ubiquitination of WT-IP6K2, whereas the mutant is resistant to ubiquitination and hence unaffected by TBB treatment. G. Model showing CK2 phosphorylation mediated ubiquitination and degradation of IP6K2.