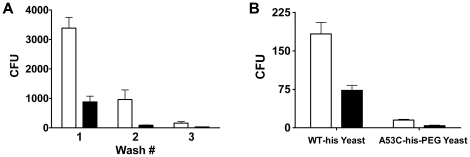
Figure 5. Human antibody binding.
The WT-his (white bars) and A53C-his-PEG (black bars) protein targets were biotinylated and captured on the surface of streptavidin coated magnetic beads. A) The two bead preparations were independently incubated with a yeast surface displayed scFv antibody library derived from human immune cells. Following binding, the beads were magnetically separated and washed three times. The number of yeast that remained bound after each wash step was determined by plating serial dilutions of the resuspended beads and enumerating cfu's. The resulting yeast colonies represent human scFvs that specifically bound to the A1-III enzymes on the corresponding magnetic beads. A53C-his-PEG coated magnetic beads bound up to 13-fold fewer human antibodies than did the WT-his coated beads (p<0.01 for each of the three washes). B) Characterization of first round binders from both protein targets. Yeast isolated as binders to either WT-his or A53C-his-PEG were propagated and subsequently incubated with magnetic beads bearing each protein target. For both yeast populations, the A53C-his-PEG coated beads (black bars) bound at least 60% fewer cells than did the WT-his beads (white bars), a result that demonstrates PEGylation effectively blocked key immunogenic epitopes (p<0.01 for all differences). Error bars represent standard deviation.