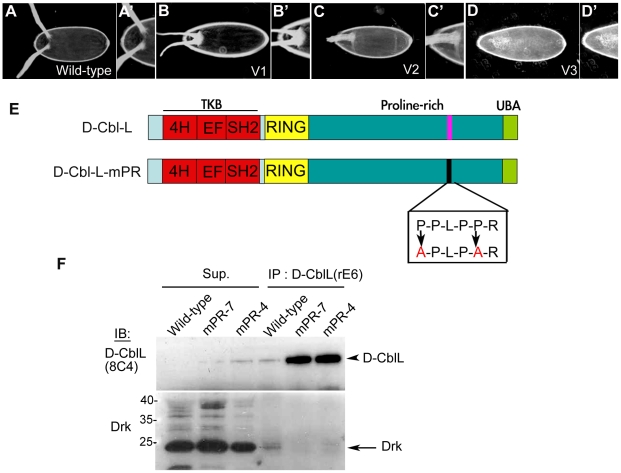
Figure 1. D-CblL directly interacts with Drk through its PR motif.
(A and A′) The EGFR activity is correlated with the morphology of dorsal appendages. Two dorsal appendages indicate normal activity of EGFR (wt). (B and B′) Two dorsal appendages fused at the base indicate low level of EGFR activity (V1), whereas (C and C′) one fused dorsal appendage indicates a even lower level of EGFR activity (V2). (D and D′) No appendage indicates the lowest level of EGFR activity (V3). (E) The schematic structure shows the wild-type D-CblL, which contains the TKB domain in its N-terminus (red), the RING finger domain (yellow) and the proline-rich domain in the C-terminus. The predicated binding motif of Drk on D-CblL is PPLPPR (shown in purple). The first and fifth prolines of the PR motif on D-CblL-mPR mutant were replaced by alanines (shown in Box). (F) The D-CblL was immunoprecipitated from OreR (wild-type) or two hs83-D-cblL-mPR; cblF165 rescued fly lines (line 4 and line 7) by the anti-D-CblL antibody (rE6). The immunoprecipitates (IP-10 µl out of 30 µl) were separated by the SDS-PAGE, and detected by the anti-D-CblL antibody (8C4) and the anti-Drk antibody. Supernatants (Sup.) were produced after immunoprecipitating, indicating the ability of immunoprecipitation in this experiment. 30 µl out of 400 µl total lysate was analyzed by western blotting in each lane. The Drk signal is near 24KD (arrow).