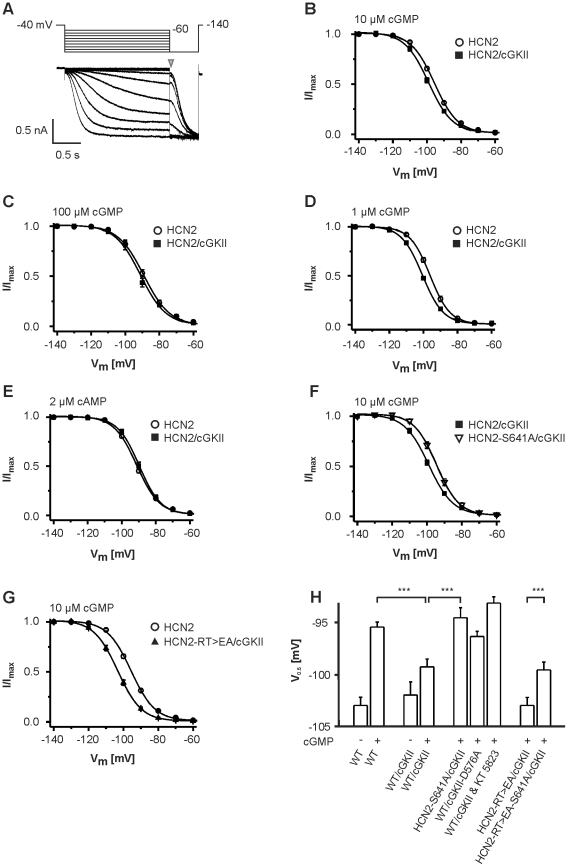
Figure 4. Regulation of voltage-dependence of HCN2 activation by cGKII.
(A) Voltage step protocol and family of current traces of a HEK293 cell transiently transfected with HCN2. (B–D) Normalized current-voltage (IV) dependence of HCN2 activation in the presence and absence of cGKII. The voltage-dependence was determined in the presence of 10 µM intracellular cGMP (B), 100 µM intracellular cGMP (C) and 1 µM intracellular cGMP (D). (E) IV curves of HCN2 in the presence or absence of cGKII at 2 µM intracellular cAMP. (F) IV curves determined at 10 µM intracellular cGMP from cells coexpressing cGKII and HCN2 or HCN2-S641A. (G) IV curves of HCN2 compared to the IV curve of an HCN2 mutant with functionally impaired cyclic nucleotide binding domain (HCN2-RT>EA) that was coexpressed with cGKII. Currents were measured in the presence of 10 µM cGMP. (H) Comparison of midpoint potentials (V0.5) of wild type (WT) and HCN2 mutants (HCN2-S641A, HCN2-RT>EA). Channels were expressed alone or together with either wild type or catalytically inactive GKII (cGKII-D576A). V0.5 was determined from the normalized IV curves in the presence (+) or absence (−) of 10 µM cGMP as indicated. In one set of experiments the cGKII was inhibited by the pharmacological blocker KT5823. *** = p<0.001.