Abstract
Domestication affects behavioral and vocal responses, involved in communication with humans; in particular, those that attract human attention. In this study, we found that silver foxes of Tame strain, experimentally domesticated for a few tenses generation, displayed bursts of vocal activity during the first minute after appearance of an unfamiliar human, that faded quickly during the remaining time of the test, when the experimenter stayed passively before the cage. Distinctively, foxes of Aggressive strain, artificially selected for tenses generations for aggressive behavior toward humans, and the control group of Unselected for behavior silver foxes kept steady levels of vocal activity for the duration of the tests. We found also that Aggressive foxes vocalized for a larger proportion of time than Unselected foxes for all five minutes of the test. We discuss the obtained data in relation to proposal effects of domestication on mechanisms directed to involving people into human-animal interactions and structural similarity between human laughter and vocalization of Tame foxes.
Keywords: Vulpes vulpes, human-animal interaction, acoustic communication, vocalization, domestication, human-exposure test
Introduction
There is a widespread interest in visual or acoustic communication between domesticated animals and humans (reviews: Hare & Tomasello, 2005; Wynne et al., 2008; Udell et al., 2010). Among subject species of these studies, most underwent a full-scale process of domestication for thousands generations, as domestic dogs Canis familiaris (Miklosi et al., 2003; Hare & Tomasello, 2005; Molnar et al., 2006; Pongracz et al., 2005, 2006; Udell et al., 2008; Viranyi et al., 2008; Wynne et al., 2008; Hare et al., 2010), domestic cats Felis catus (Nicastro & Owren, 2003; Nicastro, 2004; McComb et al., 2009) and horses Equus caballus (Hausberger et al., 2008), and some were domesticated experimentally for only a few tenses generations, as rats Rattus norvegicus (Albert et al., 2008) and Belyaev's silver foxes Vulpes vulpes (Trut, 1999; Hare & Tomasello, 2005; Hare et al., 2005).
Many authors argue that domesticated animals are able to receive and discern communicative signals from humans and that this their ability evolved as an adaptation to the tight coexistence and interaction with people (Hare & Tomasello, 2005; Viranyi et al., 2008; Trut et al., 2009; Hare et al., 2010). However, a process of animal-human communication seems to be bidirectional, representing as a mutual assessment-management (Owings & Morton, 1998). Besides receiving and discerning information from people, domesticated animals also can provide information to people, thus involving them to inter-species communication. However, the evidence of development and successfulness of such a feedback between animals and humans is rather scarce. For instance, dogs exposed to insoluble tasks, appeal for help to humans, staring on them and producing specific movements (Miklosi et al., 2003) and acoustic signals (Volodina et al., 2006). Similar tests, conducted for domestic cats, showed that humans were able to discern urgency of soliciting in purring (McComb et al., 2009), but not in meows (Nicastro & Owren, 2003).
A population of Belyaev's silver foxes, selected either for tame behaviour (Tame strain) or for enhanced aggressiveness toward humans (Aggressive strain) (Belyaev, 1979; Trut, 1999; Trut et al., 2009; Kukekova et al., 2008) provide a possibility to compare vocal behavior of animals, desiring to establish friendly animal-human contact with those of animals desiring to avoid contacts of to contact aggressively. Tame foxes, selected for friendliness to people, display emotionally-positive responses to any human. During handling, Tame foxes even grip human's hand weakly by teeth, to not give them gone (Trut, 1999). In contrast, Aggressive foxes and Unselected for behavior control group of silver foxes, respond emotionally negatively to all humans (Trut, 1999; Gogoleva et al., 2008, 2010a,c; Trut et al., 2009). The precise knowledge of attitudes of foxes of each strain to approaching humans is coming from behavioral tests (Trut, 1980, 1999; Kukekova et al., 2008; Gogoleva et al., 2009). This is supported by data coming from cross fostering, cross breeding and embryo transplantation experiments, suggesting that behavioral differences between Tame and Aggressive foxes are genetically determined (Trut, 1980, 2001). Distinctive to Tame and Aggressive foxes, Unselected for behaviour farmed silver foxes normally show aggressive-fearful responses to humans and try to increase their distance from an approaching human (Pedersen, 1994; Trut, 1999; Kukekova et al., 2008; Gogoleva et al., 2010a).
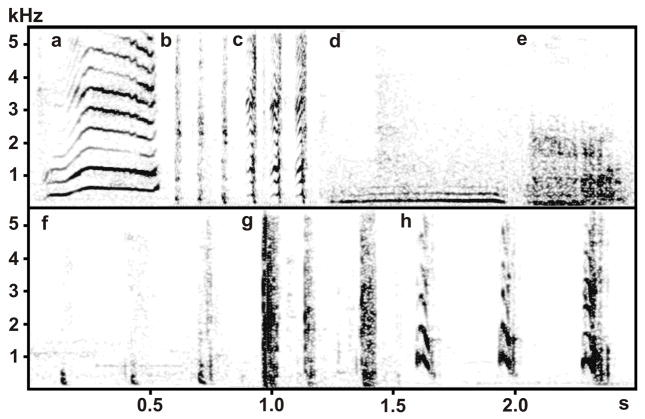
Gogoleva et al. (2008) described eight call types produced by Tame, Aggressive and Unselected silver foxes toward people: five voiced (whine, moo, cackle, growl and bark), and three unvoiced (pant, snort and cough) (Fig. 1). In the presence of humans, Tame foxes produced cackles and pants but never coughed or snorted, while Aggressive foxes produced coughs and snorts but never cackled or panted (Gogoleva et al., 2008, 2009). The control group of Unselected foxes produced cough and snort toward humans similarly to Aggressive foxes (Gogoleva et al., 2008). At the same time, vocal responses toward conspecifics were very similar between Tame, Aggressive and Unselected fox strains (Gogoleva et al., 2010a).
Figure 1.
Spectrogram illustrating call types of silver foxes toward an approaching human: (a) whine of a Tame fox, (b) pant of a Tame fox, (c) cackle of a Tame fox, (d) moo of an Unselected fox, (e) growl of an Unselected fox, (f) snort of an Aggressive fox, (g) cough of an Aggressive fox, (h) bark of an Aggressive fox.
During our previous work with silver foxes we noticed that Tame foxes, distinctive to Aggressive and Unselected foxes, showed explosive responses on the appearance of humans before their cages, as in movemental affiliative behavior (Trut, 1999; Trut et al., 2009) and in vocal reactions (Gogoleva et al., 2010b). Such behavior is similar to those of domestic dogs (Coppinger & Coppinger, 2001) and, probable, represents a mechanism directed to involvement of humans into interaction with an animal. Here we expose Tame, Aggressive and Unselected vixens to presence of a static, unfamiliar, non-responding human person. We examine quantitatively their vocal responses and discussing proposal effects of domestication on the development of mechanisms directed to involving humans into animal-human interactions.
Materials and methods
Subjects and Study Site
Our subjects were 45 adult female silver foxes, aged from 1 to 5 years, kept at the experimental fur farm of the Institute of Cytology and Genetics, Novosibirsk, Russia, as described in Gogoleva et al. (2009). Three study groups included 15 Tame (selected for tameness toward humans, 45–46 generations since the start of selection), 15 Aggressive (selected for aggressiveness toward humans; 36 generation since the start of selection) and 15 Unselected for behavior vixens. The foxes were kept and tested in individual outdoor cages 70 x 85 x 90 cm with wire mesh floor. The cages were arranged in batteries of 50 cages per row, with two rows opposite each other and 1.7 m wide passageway between them (see details in Gogoleva et al., 2009). As early exposure to humans can affect the further reactions of foxes to people, it is forbidden to pet any particular fox on this experimental farm. Fox pups socialize with conspecifics when they live together with their mothers until weaning and then live together with their littermates up to separation into individual cages at the age of 3 months. After separation they remain in visual, olfactory and auditory contact with foxes from neighboring and opposing cages. This holding regime has been standardized since 1960 and is uniform for all foxes on the farm, thereby excluding the influence of new factors on the behavior of these animals.
Dates of Work and Experimental procedure
The same researcher (S.S.G), unfamiliar to the foxes, performed all human-exposure tests (one per fox), with parallel acoustic recordings. Focal foxes were tested in June 2007, out of breeding or pup raising seasons. Each test lasted 5 minutes, and started at the moment of the researcher's approach to a focal fox cage at a distance of 50 cm. For the duration of the 5-minute-long test trial, the experimenter has been stayed at a constant distance of 50 cm from the cage front door and performed smooth hand movements left to right, not entering into interaction with a fox. Thus, the human impact on a focal animal was at the same level for the duration of the test.
Acoustic recording
We used a Marantz PMD-222 (D&M Professional, Kanagawa, Japan) cassette recorder with an AKG-C1000S (AKG-Acoustics Gmbh, Vienna, Austria) cardioid electret condenser microphone, and Type II chrome audiocassettes EMTEC-CS II (EMTEC Consumer Media, Ludwigshafen, Germany). The system had a frequency response of 0.04–14 kHz at a tape speed of 4.75 mm/s. Distance to microphone varied by 25–100 cm; the orientation of an animal to the microphone was mostly frontal or lateral. If a non-focal fox called simultaneously with the focal one, the calls of the focal fox were labeled by voice. The labeling of calls by voice is a traditional practice that is necessary in the event that more than one animal calls simultaneously. This technique allows calls of focal and other animals to be distinguished during the following analysis. The voice labeling was soft and emotionally neutral and not addressed to a focal animal. Animals of different strains are kept in interspersion, not in separate cage batteries; so the frequency of voice labeling was at the same level for all the tested strains and could not affect noticeable the results of comparison between strains.
Acoustic analysis
Successive call digitizing (with each test minute taken as a separate file) at 22.05 kHz sampling rate, 16 bit precision; high-pass filtration at 0.1 kHz and measurements were made with Avisoft-SASLab Pro v. 4.33 (Avisoft Bioacoustics, Berlin, Germany). Spectrograms for analysis were created using Hamming window, FFT-length 1024 points, frame 50%, and overlap 87.5%. By spectrogram, one researcher (S.S.G) classified each call visually to one of eight types according to the vocal traits described in Gogoleva et al. (2008, 2009), blindly to the strain to which a fox belonged to. In total, we examined 5,027 calls: 1,976 calls from Tame foxes, 1,936 calls from Aggressive foxes and 1,115 calls from Unselected foxes.
To calculate the proportion of time spent vocalizing per fox per test minute, i.e., the total duration of calls within each minute (taken in %), we measured the duration of each call within each minute, with the standard marker cursor in the main window of Avisoft software. The measurements were exported automatically to Excel (Microsoft Corp., Redmond, WA, USA). For each fox, the calling rates (calls/min), were calculated as the number of calls per each minute of the test.
Statistics
All statistical analyses were carried out with STATISTICA, v. 6.0 (StatSoft, Inc., Tulsa, OK, USA). All tests were two-tailed, and differences were considered significant where p<0.05. We applied parametrical tests, as a Kolmogorov-Smirnov test showed that distributions of the variable values did not depart from normality (p>0.05) in all comparisons. We used one-way repeated measures ANOVA with Newman-Keuls post hoc tests to compare the vocal traits between the test minutes. In addition, we used GLM for repeated measures, with successive number of test minute as the repeated factor and strain as the fixed factor, with the Newman-Keuls as post hoc to compare the vocal traits between strains. We also used a χ2 test with Fisher exact post hoc test, to compare the proportions of calls of different types between the first and remaining minutes of the test.
Results
Only whine, moo and growl were found in all strains (Fig. 2). Tame foxes also produced cackle and pant but not cough and snort. In contrast, Unselected and Aggressive foxes also produced cough and snort, but not cackle and pant. Bark was the rarest vocalization, found only in two Aggressive foxes. The numbers of growls and whines in the Unselected strain, of growls and barks in the Aggressive strain and of growls and moos in the Tame strain were too small to allow inclusion of these call types into analyses.
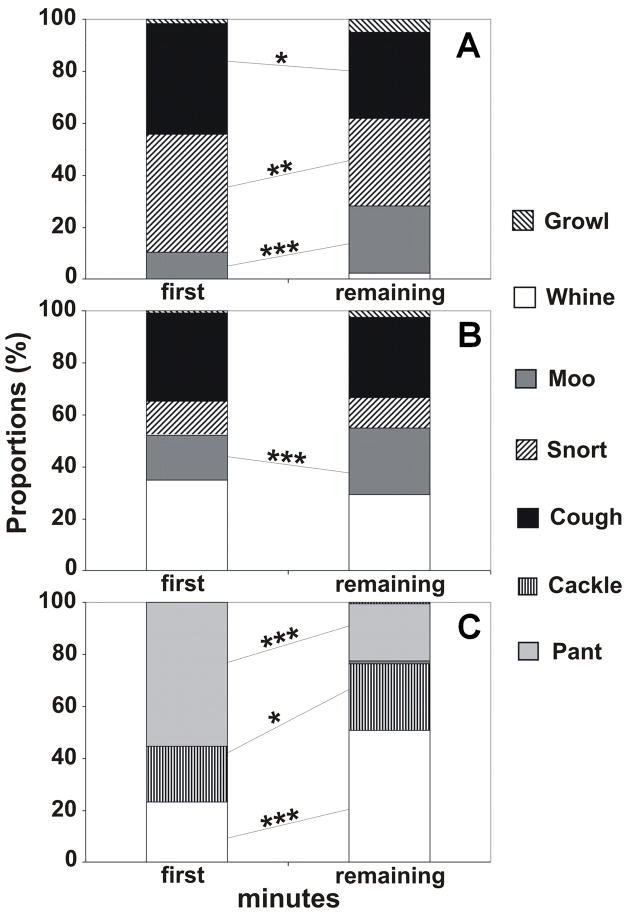
Figure 2.
Proportions of calls of different types for the first and the remaining minutes of a human-exposure test applied to Unselected (A), Aggressive (B) and Tame (C) silver foxes and comparison between the minutes with Fisher exact test: * - p < 0.05; ** - p < 0.01; *** - p < 0.001.
Unselected, Aggressive and Tame foxes showed distinctive trends for the proportions of different call types between the first and remaining minutes of the test (Fig. 2). The χ2 tests showed that proportions of calls of different types differed significantly between first and remaining minutes in all three strains (Unselected: χ2 = 33.1, df = 2, p<0.001; Aggressive: χ2 = 14.0, df = 3, p<0.01; Tame: χ2 = 238.6, df = 2, p<0.001). In Unselected foxes, a Fisher exact post hoc test showed that the proportion of moos increased significantly and the proportions of coughs and snorts decreased significantly between first and remaining minutes (Fig. 2). In Aggressive foxes, only the proportion of moos increased significantly between first and remaining minutes. In Tame foxes, the proportions of whines and cackles increased significantly and the proportion of pants decreased significantly between first and remaining minutes (Fig. 2).
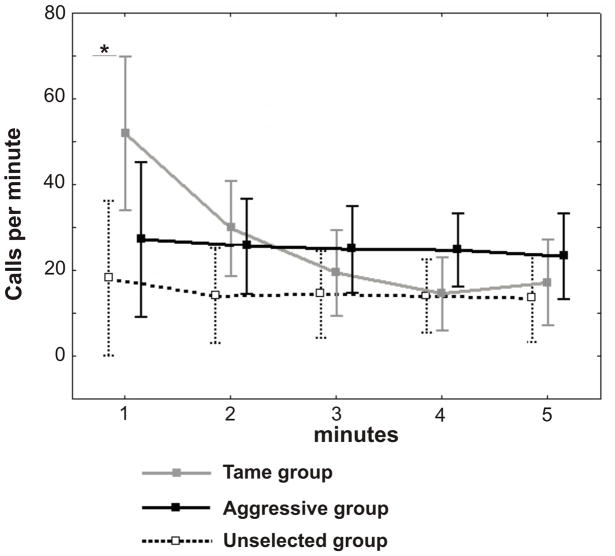
A one-way repeated measures ANOVA revealed a significant effect of the test minute on the calling rate in Tame foxes (F4,56 = 4.74, p=0.002), but not in Aggressive (F4,56 = 0.39, p=0.81) or Unselected foxes (F4,56 = 0.95, p=0.44) (Fig. 3). For the calling rate of Tame foxes, Newman-Keuls post hoc test showed a significant decrease between a first minute and remaining minutes (p=0.03 between 1st and 2nd minutes and p<0.01 between 1st and 3rd, 1st and 4th, 1st and 5th minutes respectively). Therefore, the trends of the calling rate throughout the test differed significantly between strains (GLM for repeated measures, F8,168 = 3.28, p=0.002), showing an abrupt decrease between a first minute and all subsequent minutes in Tame foxes, and a constant level throughout the test maintained in Aggressive and Unselected foxes. Between strains, only first minutes differed in the calling rate, found significantly higher in Tame foxes than in Unselected foxes (Newman-Keuls post hoc test, p=0.028) (Fig. 3).
Figure 3.
The values of the calling rate occurring at five successive minutes of a human-exposing test, applied to Tame (grey solid line), Aggressive (black solid line) and Unselected (dashed line) foxes. Central points show means, whiskers show 0.95 confidence intervals. Asterisks show significant differences (p<0.05) between Tame and Unselected foxes (post hoc Newman-Keuls test).
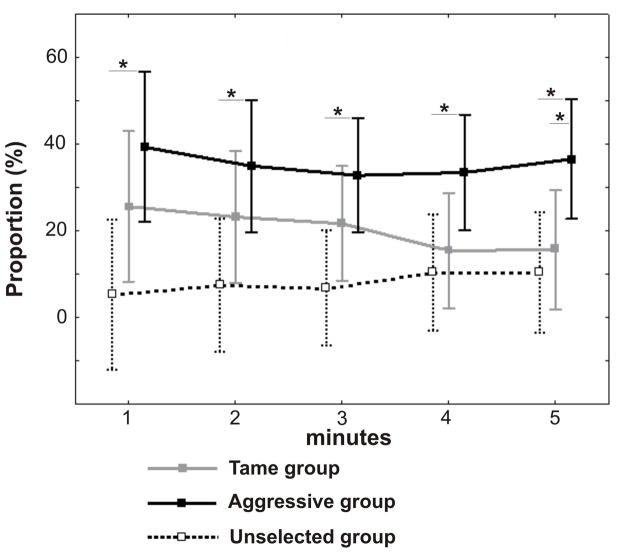
Distinctive to the values of the calling rate, the values of the proportion of time spent vocalizing varied between strains but not in trends throughout the test (Fig. 4). A one-way repeated measures ANOVA did not reveal significant effects of the test minute on the proportion of time spent vocalizing in Tame (F4,56 = 1.38, p=0.25), Aggressive (F4,56 = 0.51, p=0.73) or Unselected foxes (F4,56 = 2.33, p=0.07). Thus, the trends of the proportion of time spent vocalizing throughout the test did not differ significantly between strains (GLM for repeated measures, F8,168 = 1.31, p=0.24), showing uniformly constant run in the Tame, Aggressive and Unselected foxes. However, the values of the proportion of time spent vocalizing were significantly higher in Aggressive foxes than in Unselected foxes, on all the five minutes of the test (Newman-Keuls post hoc test, p<0.05 in all comparisons), and than in Tame foxes, on the 5th minute of the test (Newman-Keuls post hoc test, p<0.05) (Fig. 4).
Figure 4.
The values for the proportion of time spent vocalizing for five successive minutes of a human-exposing test, applied to Tame (grey solid line) Aggressive (black solid line) and Unselected (dashed line) foxes. Central points show means, whiskers show 0.95 confidence intervals. Asterisks show significant differences (p<0.05) between Aggressive and Unselected and between Aggressive and Tame foxes (post hoc Newman-Keuls test).
Discussion
Only experimentally domesticated Tame foxes showed the explosion and fading of vocal activity to an approach and presence of the unfamiliar human. We can propose that the fading was due to non-responsiveness of the experimenter to the animal soliciting to interact. In contrast, the Aggressive and Unselected foxes displayed permanent vocal activity for the duration of the test, maintaining constant levels of the calling rate and proportion of time spent vocalizing.
Consistently to results of previous studies (Gogoleva et al., 2008, 2009, 2010b,c), in the presence of humans, Unselected and Aggressive foxes produced coughs and snorts and never emitted cackles and pants, while Tame foxes produced cackles and pants and never emitted coughs or snorts. Also, in accordance to previous data for comparison of vocal activities of these three strains under other test designs (Gogoleva et al., 2008, 2010b), Aggressive foxes showed higher proportions of time spent vocalizing throughout the test than Unselected or Tame foxes. Therefore, both the use of different call types and the proportion of time spent vocalizing toward humans represent sustainable characteristics of these three fox strains independent on test design.
Although Unselected and Aggressive foxes showed changes in proportions of different call types between the first and remaining minutes of the test, in Tame foxes these changes were more noticeable. From the first to remaining minutes of the test in Tame foxes the proportion of pants decreased, with a respective increase in the proportion of whines more than twice. This resulted in significant decrease of calling rate, but did not change the value of vocal activity, as larger number of short pants (42 ± 9 ms of duration) was compensated with less number of longer whines (711 ± 502 ms of duration, see Gogoleva et al., 2008).
The calling rate represents a reliable indicator of the degree of emotional arousal in silver foxes (Gogoleva et al., 2010b,c) and other mammals (Weary & Fraser, 1995; Weary et al., 1997; Rendall, 2003; Volodin et al., 2009). Therefore, we can suggest reasonable that the human appearance before the cage provokes the high level of emotional arousal of Tame foxes. Besides, Tame foxes respond to human approach with affiliative displays (mouth ajar, wagging the tail, moving on half-bent paws, turning occasionally onto side or back, or even belly up), similarly to domestic dogs (Trut, 1999; Coppinger & Coppinger, 2001; Trut et al., 2009). These vocal and non-vocal displays suggest that Tame foxes wish to interact to humans. However, the absence of response from the experimenter results in quick decrease of behavioral advertising and calling rate as soon as at a second minute of the test. A previous study (Gogoleva et al., 2010b) showed that only tactile contacts, caressing and handling were more stimulating for Tame foxes compared to the human appearance before a fox cage.
Also, hormonal responses suggest that Tame foxes do not experience stress when contact with humans. A comparative study of functioning of hypothalamo-pituitary-adrenal axis and neurotransmitter serotonin system in different strains of silver foxes showed that in Tame foxes (45 generation since the start of selection) basal and stress-induced blood cortisol levels were respectively three- and five-fold lower than in the Unselected foxes (Oskina et al., 2008; Trut et al., 2009). Studies of the brain’s serotonin system shown, that Tame foxes differ from the Unselected foxes by higher levels of serotonin and its main metabolite 5-hydroxyindol acetic acid, in a number of brain structures (Popova et al., 1991; Trut et al., 2009).
Thus, bursts of vocal activity as a component of affiliative behavior of domesticated Tame foxes could result from positive emotional state arising from interactions with people. Thus, as for pets, for Tame silver foxes, directionally selected for many generations for tolerance to people, humans represent a source of positive emotions. We can propose, that the revealed in this study very high calling rate in response to human appearance near the cage may act as behavioral mechanism for attraction of human attention, that is directed to prolonging the contact and involving the human into animal-human interaction. In Tame foxes, this behavior is registered as early as at the age of one month, i.e. during their first testing for interaction with humans (Trut, 1999). Probable, artificial selection for tameness determines the trigger of this kind of behavior, directed on the attraction of attention already during the first interaction with a human. The positive feedback may arise and establish on the base of operant conditioning during further repeated tests for behavioral scoring (Trut, 1999; Trut et al., 2009). If the behavior directed to attracting attention is not responded by humans, it fades.
Of especial interest is similarity in the structure of human laughter with the structure of fox cackles and pants, that is, calls producing toward humans exclusively by Tame foxes (Supplementary audio file). Cohen & Fox (1976) supposed that pant represents an invitation to play in domestic dogs and red foxes and proposed an interesting analogy between pant in the domestic dog and the laughing in humans, since similar a special facial expression, semi-open mouth and concomitant pant, occur during invitation to play in both species. Similarly to voiced cackles and unvoiced pants (Gogoleva et al., 2008), the laughter also can be voiced and unvoiced (Bachorowski et al., 2001). The laughter is produced in bouts, with average rate of 4.37 calls per second (Bachorowski et al., 2001). Cackles and pants are also produced in bouts, with average rate of 4.76 cackles per second and 5.56 pants per second (Gogoleva et al., 2008). Duration of all the three call types is short, consisting 110 ± 80 ms for the voiced laughter, 60 ± 10 ms for cackles and 42 ± 9 ms for pants. The ranges of fundamental frequency of the voiced laughter in women (355 – 415 Hz) and of the voiced cackles of vixens (330 – 490 Hz) are also very close to each other (Bachorowski et al., 2001; Gogoleva et al., 2008). Such surprising similarity in the structure of calls of Tame foxes toward humans and of human laughter can explain high responsiveness of people to affiliative behavior of Tame foxes and easiness of involving humans in interactions with them (Trut, 1999). These findings are consistent to the reported by McComb et al. (2009) use by domestic cats of purring with tonal component similar in fundamental frequency to those of human infant’s cry, for food soliciting in their owners. Probable, that vocal communication of Tame foxes with humans also represents a kind of exploitation of sensory biases of receivers (Owings & Morton, 1998) providing animal signalers an effective mean of managing humans (McComb et al., 2009).
However, there is no consensus whether mechanisms of animal-human interactions are innate or developmental. The "innate" hypothesis is based on reports that dog pups use human pointing gestures for searching hidden food items as early as at 6–9 weeks of age, whilst undomesticated timber wolves Canis lupus and chimpanzee Pan troglodytes are unable to resolve this task without special training (Hare & Tomasello, 2005; Hare et al., 2006; Viranyi et al., 2008). Consistently with this hypothesis, experimentally domesticated Tame silver foxes perform this task already as pups, while undomesticated Unselected silver foxes fail to perform it (Hare et al., 2005). The "developmental" hypothesis is based on reports that wolves perform this task better than dogs and that dog performance is improved with age (Wynne et al., 2008; Udell & Wynne, 2010). Abilities of wolves to use human pointing gestures also improved with special training (Viranyi et al., 2008). Ontogenesis of vocal behavior of Tame silver foxes should be studied more thoroughly to reveal what innate and ontogenetic mechanisms are involved in vocal and non-vocal displays directed to attraction human attention and human-animal interactions.
Supplementary Material
Acknowledgments
We would like to thank the staff of the experimental fur farm of the Institute of Cytology and Genetics RAS, Novosibirsk, Russia, for their help and support. During our work, we adhered to the “Guidelines for the treatment of animals in behavioural research and teaching” (Anim. Behav., 2006, 71, 245–253) and to the laws of Russian Federation, the country where the research was conducted. The compliance of husbandry conditions and use of animals for research PHS Policy on Humane Care and Use of Laboratory Animals has been approved by Public Health Service (PHS) Assurance for the Institute of Cytology and Genetics (license number A5761-01). This study was supported by the Russian Foundation for Basic Research grant 09-04-00416 (for SG, IV and EV), by National Institutes of Health grants R03 TW008098-01 and R01 MH077811, and the Programs of Basic Research of the RAS Presidium “Biodiversity and gene pool dynamics” and “Molecular and Cell Biology” (for AK and LT).
Appendix A. Supplementary data
Supplementary audio file contains a call bout of a Tame fox, starting with pants and ending with cackles.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert FW, Shchepina O, Winter C, Römpler H, Teupser D, Palme R, Ceglarek U, Kratzsch J, Sohr R, Trut LN, Thiery J, Morgenstern R, Plyusnina IZ, Schöneberg T, Pääbo S. Phenotypic differences in behavior, physiology and neurochemistry between rats selected for tameness and for defensive aggression towards humans. Hormones and Behav. 2008;53:413–421. doi: 10.1016/j.yhbeh.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Bachorowski JA, Smoski MJ, Owren MJ. The acoustic features of human laughter. J Acoust Soc Am. 2001;110:1581–1597. doi: 10.1121/1.1391244. [DOI] [PubMed] [Google Scholar]
- Belyaev DK. Destabilizing selection as a factor in domestication. J Hered. 1979;70:301–308. doi: 10.1093/oxfordjournals.jhered.a109263. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Fox MW. Vocalizations in wild canids and possible effects of domestication. Behav Process. 1976;1:77–92. doi: 10.1016/0376-6357(76)90008-5. [DOI] [PubMed] [Google Scholar]
- Coppinger R, Coppinger L. Dogs: A Startling New Understanding of Canine Origin, Behavior, and Evolution. Scribner; New York: 2001. [Google Scholar]
- Gogoleva SS, Volodin IA, Volodina EV, Kharlamova AV, Trut LN. Kind granddaughters of angry grandmothers: the effect of domestication on vocalization in cross bred silver foxes. Behav Process. 2009;81:369–375. doi: 10.1016/j.beproc.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogoleva SS, Volodin IA, Volodina EV, Kharlamova AV, Trut LN. Vocalization toward conspecifics in silver foxes (Vulpes vulpes) selected for tame or aggressive behavior toward humans. Behav Process. 2010a;84:547–554. doi: 10.1016/j.beproc.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogoleva SS, Volodin IA, Volodina EV, Kharlamova AV, Trut LN. Sign and strength of emotional arousal: Vocal correlates of positive and negative attitudes to humans in silver foxes (Vulpes vulpes) Behaviour. 2010b;147 doi: 10.1163/000579510X528242. [DOI] [Google Scholar]
- Gogoleva SS, Volodin IA, Volodina EV, Trut LN. To bark or not to bark: Vocalization in red foxes selected for tameness or aggressiveness toward humans. Bioacoustics. 2008;18:99–132. [Google Scholar]
- Gogoleva SS, Volodina EV, Volodin IA, Kharlamova AV, Trut LN. The gradual vocal responses to human-provoked discomfort in farmed silver foxes. Acta Ethol. 2010c;13 doi: 10.1007/s10211-010-0076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare B, Call J, Tomasello M. Chimpanzees deceive a human competitor by hiding. Cognition. 2006;101:495–514. doi: 10.1016/j.cognition.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Hare B, Plyusnina I, Ignacio N, Schepina O, Stepika A, Wrangham R, Trut L. Social cognitive evolution in captive foxes is a correlated byproduct of experimental domestication. Current Biol. 2005;16:226–230. doi: 10.1016/j.cub.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Hare B, Rosati A, Kaminski J, Braeur J, Call J, Tomasello M. The domestication hypothesis for dogs’ skills with human communication: a response to Udell et al. (2008) and Wynne et al. (2008) Anim Behav. 2010;79:e1–e6. [Google Scholar]
- Hare B, Tomasello M. Human-like social skills in dogs? Trends Cogn Sci. 2005;9:439–444. doi: 10.1016/j.tics.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Hausberger M, Roche H, Henry S, Visser EK. A review of the human–horse relationship. Appl Anim Behav Sci. 2008;109:1–24. [Google Scholar]
- Kukekova AV, Trut LN, Chase K, Shepeleva DV, Vladimirova AV, Kharlamova AV, Oskina IN, Stepika A, Klebanov S, Erb HN, Acland GM. Measurement of segregating behaviors in experimental silver fox pedigrees. Behav Genet. 2008;38:185–194. doi: 10.1007/s10519-007-9180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComb K, Taylor AM, Wilson C, Charlton BD. The cry embedded within the purr. Current Biol. 2009;19:R507–R508. doi: 10.1016/j.cub.2009.05.033. [DOI] [PubMed] [Google Scholar]
- Miklósi A, Kubinyi E, Topal J, Gacsi M, Virányi Z, Csanyi V. A simple reason for a big difference: wolves do not look back at humans, but dogs do. Current Biol. 2003;13:763–766. doi: 10.1016/s0960-9822(03)00263-x. [DOI] [PubMed] [Google Scholar]
- Molnár C, Pongrácz P, Dóka A, Miklósi Á. Can humans discriminate between dogs on the base of the acoustic parameters of barks? Behav Process. 2006;73:76–83. doi: 10.1016/j.beproc.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Nicastro N. Perceptual and acoustic evidence for species-level differences in meow vocalizations by domestic cats (Felis catus) and African wild cats (Felis silvestris lybica) J Comp Psychol. 2004;118:287–296. doi: 10.1037/0735-7036.118.3.287. [DOI] [PubMed] [Google Scholar]
- Nicastro N, Owren MJ. Classification of domestic cat (Felis catus) vocalizations by naïve and experienced human listeners. J Comp Psychol. 2003;117:44–52. doi: 10.1037/0735-7036.117.1.44. [DOI] [PubMed] [Google Scholar]
- Oskina IN, Herbeck YuE, Shikhevich SG, Plyusnina IZ, Gulevich RG. Alterations in the hypothalamus–pituitary–adrenal and immune systems during selection of animals for tame behavior. VOGiS Herald. 2008;12:39–49. (in Russian) [Google Scholar]
- Owings DH, Morton ES. Animal Vocal Communication: A New Approach. Cambridge Univ. Press; Cambridge: 1998. [Google Scholar]
- Pedersen V. Long–term effects of different handling procedures on behavioural, physiological, and production–related parameters in silver foxes. Appl Anim Behav Sci. 1994;40:285–296. [Google Scholar]
- Pongrácz P, Miklósi A, Molnár C, Csanyi V. Human listeners are able to classify dog (Canis familiaris) barks recorded in different situations. J Comp Psychol. 2005;119:136–144. doi: 10.1037/0735-7036.119.2.136. [DOI] [PubMed] [Google Scholar]
- Pongrácz P, Molnár C, Miklósi A. Acoustic parameters of dog barks carry emotional information for humans. Appl Anim Behav Sci. 2006;100:228–240. [Google Scholar]
- Popova N, Voitenko N, Kukikov A, Avgustinovich D. Evidence for the involvement of central serotonin in the mechanism of domestication of silver foxes. Pharmacol Biochem Behav. 1991;40:751–756. doi: 10.1016/0091-3057(91)90080-l. [DOI] [PubMed] [Google Scholar]
- Rendall D. Acoustic correlates of caller identity and affect intensity in the vowel like–grunt vocalizations of baboons. J Acoust Soc Am. 2003;113:3390–3402. doi: 10.1121/1.1568942. [DOI] [PubMed] [Google Scholar]
- Trut LN. The genetics and phenogenetics of domestic behavior. In: Belyaev DK, editor. Book 2: Problems of General Genetic; Proceedings of the XIV International Congress of Genetics; Moscow: MIR Publishers; 1980. pp. 123–136. [Google Scholar]
- Trut LN. Early canid domestication: the farm-fox experiment. Amer Scientist. 1999;87:160–169. [Google Scholar]
- Trut LN. Experimental studies of early canid domestication. In: Ruvinsky A, Sampson J, editors. The Genetics of the Dog. CABI Publishing; New York: 2001. pp. 15–41. [Google Scholar]
- Trut LN, Oskina IN, Kharlamova AV. Animal evolution during domestication: the domesticated fox as a model. BioEssays. 2009;31:349–360. doi: 10.1002/bies.200800070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udell MAR, Dorey NR, Wynne CDL. Wolves outperform dogs in following human social cues. Anim Behav. 2008;76:1767–1773. [Google Scholar]
- Udell MAR, Dorey NR, Wynne CDL. What did domestication do to dogs? A new account of dogs’ sensitivity to human actions. Biol Rev Camb Philos Soc. 2010;85:327–345. doi: 10.1111/j.1469-185X.2009.00104.x. [DOI] [PubMed] [Google Scholar]
- Udell MAR, Wynne CDL. Ontogeny and phylogeny: both are essential to human-sensitive behaviour in the genus Canis. Anim Behav. 2010;79:e9–e14. [Google Scholar]
- Virányi Z, Gacsi M, Kubinyi E, Topal J, Belenyi B, Ujfalussy D, Miklósi A. Comprehension of human pointing gestures in young human-reared wolves (Canis lupus) and dogs (Canis familiaris) Anim Cogn. 2008;11:373–387. doi: 10.1007/s10071-007-0127-y. [DOI] [PubMed] [Google Scholar]
- Volodin IA, Volodina EV, Gogoleva SS, Doronina LO. Indicators of emotional arousal in vocal emissions of the humans and nonhuman mammals. Journal of General Biology. 2009;70:210–224. (in Russian) [PubMed] [Google Scholar]
- Volodina EV, Volodin IA, Filatova OA. Advances in Bioacoustics II, Dissertationes SASA, Classis IV: Historia Naturalis. Vol. 47. 2006. The occurrence of nonlinear vocal phenomena in frustration whines of the domestic dog (Canis familiaris) pp. 257–270. [Google Scholar]
- Weary DM, Frazer D. Calling by domestic piglets: reliable signals of need? Anim Behav. 1995;50:1047–1055. [Google Scholar]
- Weary DM, Ross S, Fraser D. Vocalizations by isolated piglets: a reliable indicator of piglet need directed towards the sow. Appl Anim Behav Sci. 1997;53:249–257. [Google Scholar]
- Wynne CDL, Udell MAR, Lord KA. Ontogeny’s impact on human–dog communication. Anim Behav. 2008;76:e1–e4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.