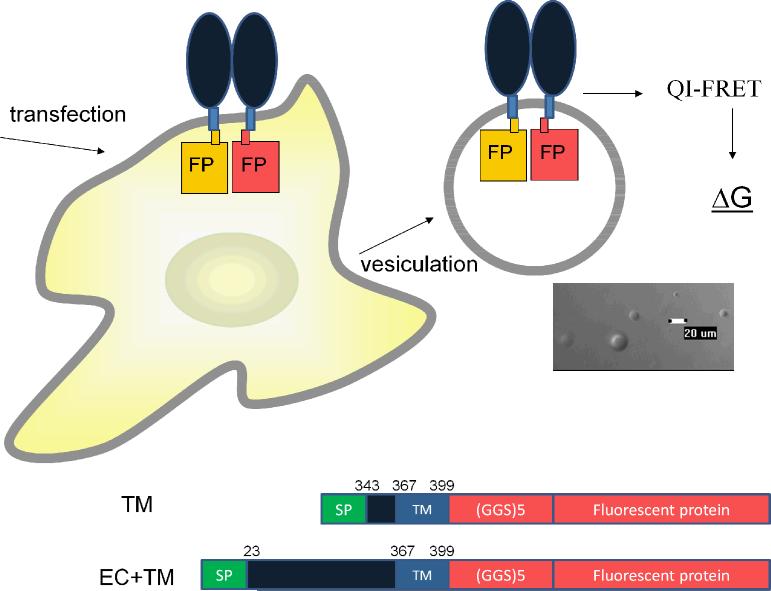
Figure 1.
Overview of measurements of dimerization free energy in plasma membrane-derived vesicles. Two different constructs were used (i) A EC+TM construct consisting of the signal peptide, the EC domain, the TM domain, a 15 amino acid-long (GGS)5 flexible linker, and the fluorescent proteins and (ii) a TM construct lacking the three Ig-like motifs of FGFR3 extracellular domain, consisting of the signal peptide, a 24 amino acid segment of the EC domain, the TM domain, a 15 amino acid (GGS)5 flexible linker, and the fluorescent proteins. We determine the contribution of the EC domain to FGFR3 dimerization by measuring the difference in dimerization free energy for these two constructs.