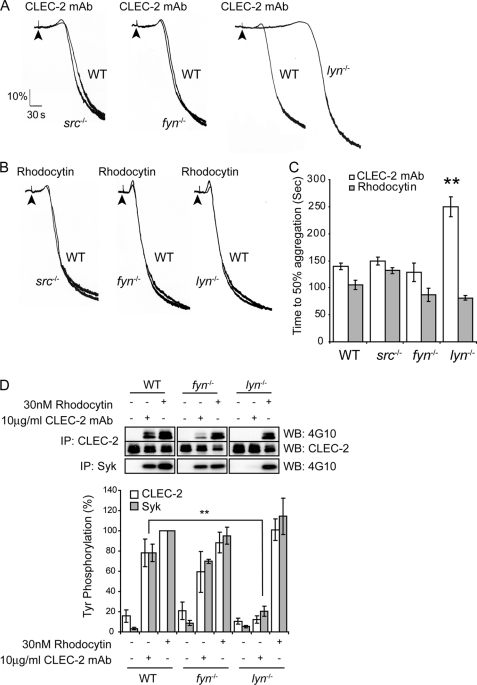
FIGURE 3.
Lyn plays a critical role in CLEC-2-mediated signaling by antibody ligation but is not involved in response to rhodocytin. Fyn-, Lyn-, or Src-deficient washed platelets and their litter-matched wild-type platelets (2 × 108 platelets/ml) were stimulated with 10 μg/ml CLEC-2 mAb (A) or 30 nm rhodocytin (B). Platelet aggregation was measured as a change in light transmission, using a lumi-aggregometer. Representative aggregation traces are shown. The addition of the agonist is indicated by an arrowhead. C, data represent the means of the time to get 50% of aggregation and standard error of three to six independent experiments. **, p < 0.005 (significant difference versus wild type, according to two-tailed Student's t test). D, Fyn- or Lyn-deficient washed platelets and their litter-matched wild-type platelets (2 × 108 platelets/ml) were stimulated with 10 μg/ml CLEC-2 mAb or 30 nm rhodocytin for 3 min. CLEC-2 and Syk were immunoprecipitated (IP), and immunoprecipitates were immunoblotted with an anti-phosphotyrosine antibody. CLEC-2 immunoprecipitates were also immunoblotted with anti-CLEC-2 antibody as described under “Experimental Procedures.” The percentage of tyrosine phosphorylation was measured at 3 min of stimulation and represented as the means and standard error of three to six independent experiments. **, p < 0.005 (significant difference versus wild type, according to two-tailed Student's t test). WB, Western blotting.