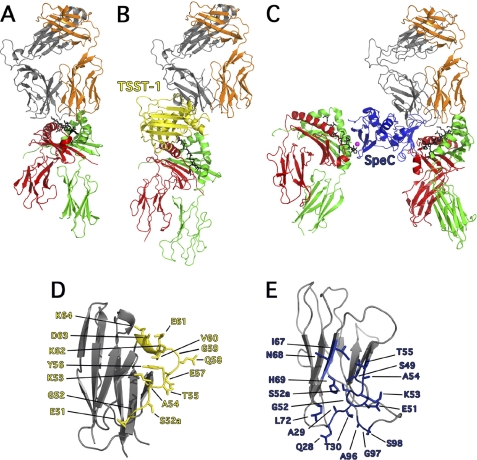
FIGURE 2.
Exposition of the differences between conventional TCR-pMHC and TCR-SAg-pMHC T cell signaling complexes. A, cartoon representation of conventional TCR-pMHC ternary complex (36), where the TCR adopts a semi-conserved diagonal docking mode on pMHC II. By and large, the CDR1 and CDR2 loops engage the α-helices of MHC II and the CDR3 loops engage the antigenic peptide. B, cartoon representation of the TSST-1-mediated T cell activation complex (20), where TSST-1 acts a bridge between TCR and pMHC II complex and direct TCR contact with pMHC II is abrogated, thus removing antigenic peptide recognition. C, cartoon representation of the SpeC-mediated T cell activation complex (11, 16, 21) where SpeC cross-links MHC II molecules through zinc-mediated, high-affinity binding to the pMHC II β-chain (left) (16), and as a wedge, by binding to the low-affinity binding site on the pMHC II α-chain (11) and preventing direct contacts between TCR Vβ and the antigenic peptide. Colors are as follows: MHC α-chain, green; MHC β-chain, red; antigenic peptide, black; TCR α-chain, orange; TCR β-chain, gray; Zn2+ ion, magenta; TSST-1, yellow and SpeC, blue. D, close up view of hVβ2.1 (PDB code 3MFG) showing all the residues involved in binding to TSST-1. E, close up view of hVβ2.1 (PDB code 1KTK) showing all residues involved in binding to SpeC.