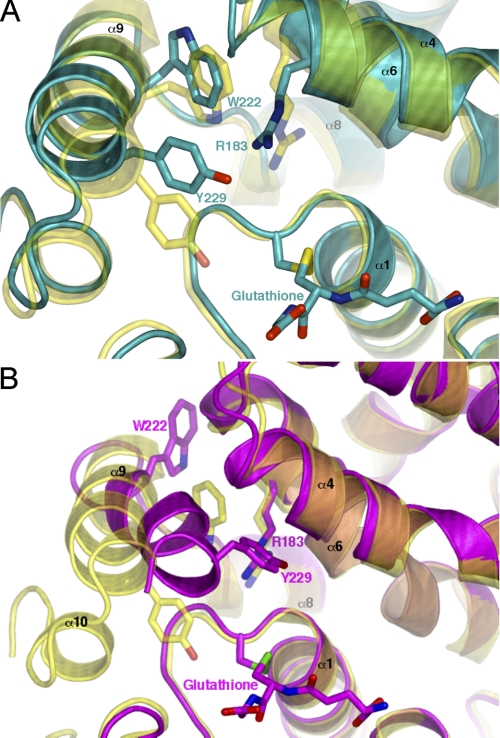
FIGURE 2.
The flexibility of helix α9 observed in the ΔGlu-155 structure. A, a closer view of the A monomer active site reveals several key differences compared with the native enzyme. Of most interest is the different rotamer adopted by Trp-222, dramatically increasing the hydrophobicity within the conserved H-site. The ΔGlu-155 A-monomer is shown in cyan compared with the wild type enzyme in yellow. B, comparison of the native structure to that of the B monomer reveals much more pronounced structural change. Helix α9 has shifted toward the site of glutathione binding by several angstroms, dramatically reducing the size of the H-site. The ΔGlu-155 B-monomer is shown in magenta with the overlaid native structure in yellow. The figures were generated using PyMol.