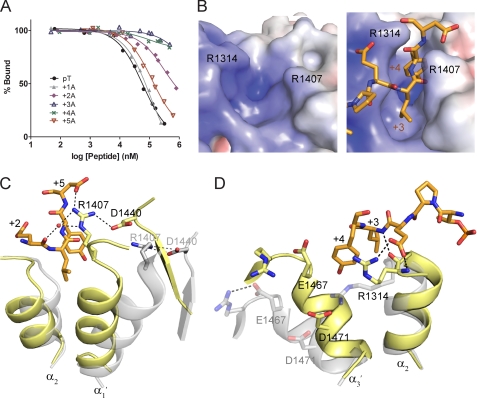
FIGURE 4.
TopBP1-BACH1 interaction at the +3/+4 binding pocket. A, FP competition analysis of the BACH1-binding motif using alanine scanning mutagenesis. BACH1 phospho-peptides mutated to alanine at +1 to +5 positions were used to compete with the FITC-labeled phospho-peptide bound to TopBP1 BRCT7/8. B, electrostatic potential surface of the TopBP1 BRCT7/8 + 3/+4 binding pocket in the apo (left) and peptide-bound (right) structures. TopBP1 Arg1314 and Arg1407 residues are mapped on the surface. C, role of Arg1407 in the TopBP1-BACH1 complex. TopBP1 BRCT7/8 in the apo (gray) and complex (yellow) structures are superimposed. Residues involved in interacting with Arg1407 are labeled. D, role of Arg1314 in +2/+3 binding of the BACH1 peptide. TopBP1 BRCT7/8 in the apo (gray) and complex (yellow) structures are superimposed.