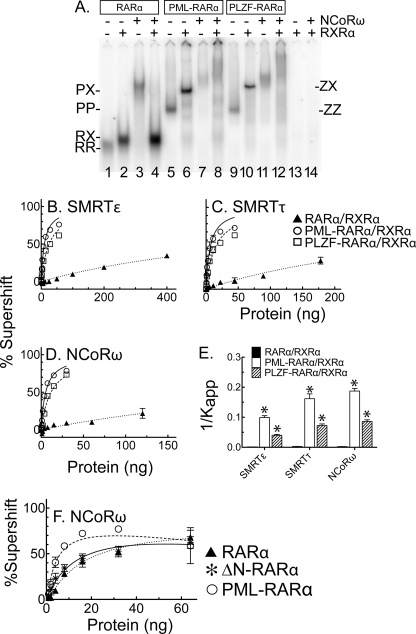
FIGURE 4.
Distinct effects of RXRα on corepressor recruitment by RARα, PML-RARα, and PLZF-RARα. A, representative EMSA supershift using the NCoRω construct. All of the reactions contained a fixed amount of 32P-labeled probe and each RARα or x-RARα protein; RXRα and/or NCoRω was included or omitted as indicated. The positions of the RARα, PML-RARα, or PLZF-RARα homodimers (labeled RR, PP, or ZZ, respectively) and RXRα heteromers (labeled RX, PX, and ZX, respectively) in the absence of corepressor are indicated. B–D, binding of RXRα heteromers of RARα, PML-RARα, or PLZF-RARα to three different corepressor variants. The experiments in Fig. 3 (A, B, and E) were repeated in the presence of RXRα; the means and S.E. are shown (n = 3). E, relative affinities of RXRα heteromers of RARα, PML-RARα, and PLZF-RARα heteromers for the different corepressor variants. The 1/Kapp values were calculated from B–D. The low affinity of RARα/RXRα for corepressor produced a poorer fit to theoretical kinetics than did the x-RARα/RXRα fusions, with R2 values of <0.9. F, corepressor binding by ΔN-RARα homodimers. EMSA supershifts were as in Fig. 3; the means and S.E. are presented (n = 2).