Abstract
Low-intensity ultrasound (LIUS) treatment has been shown to increase mass transport, which could benefit tissue grafts during the immediate postimplant period, when blood supply to the implanted tissue is suboptimal. In this in vitro study, we investigated effects of LIUS stimulation on dye diffusion, proliferation, metabolism, and tropomyosin expression of muscle cells (C2C12) and on tissue viability and gene expression of human adipose tissue organoids. We found that LIUS increased dye diffusion within adjacent tissue culture wells and caused anisotropic diffusion patterns. This effect was confirmed by a hydrophone measurement resulting in acoustic pressure 150–341 Pa in wells. Cellular studies showed that LIUS significantly increased proliferation, metabolic activity, and expression of tropomyosin. Adipose tissue treated with LIUS showed significantly increased metabolic activity and the cells had similar morphology to normal unilocular adipocytes. Gene analysis showed that tumor necrosis factor-alpha expression (a marker for tissue damage) was significantly lower for stimulated organoids than for control groups. Our data suggests that LIUS could be a useful modality for improving graft survival in vivo.
1. Introduction
Autografted adipose tissue is potentially an excellent soft tissue space-filler because it is autologous, abundant, and biocompatible. Adipose tissue autografts have been used for facial plastic surgery, treatment of burn patients, breast implants and to improve phonatory closure of vocal folds [1–5]. However, the survival of adipose grafts can be quite variable and their volume is often decreased significantly after several months. An important determinant of long-term adipose autograft survival is thought to be short-term survival during the postimplantation period. Before a new blood supply to an implanted tissue is established, diffusion limits the delivery of oxygen and glucose and the removal of metabolites including CO2 and lactate, particularly to the more central parts of a graft. These factors will impact the survival of key cell types present in the implant, including adipocytes, preadipocytes, and stem cells. The poor survival of adipose autografts reported in many studies may therefore relate to effects of limited mass transport (which by definition includes diffusion, convection, and other means of translocating molecules) during the postimplant period [6–8].
Ultrasound (US) technology has been used in biotechnology for improving of cell viability via its ability to increase mass transport [9–11]. US enhances molecular motion via thermal and nonthermal effects (cavitation, microstreaming), and has been tested previously as a means for increasing blood flow and improving tissue healing and regeneration [12, 13]. Some previous applications of low-intensity ultrasound (LIUS) have been in the context of cartilage and bone regeneration or tissue engineering, where it was shown that LIUS stimulated increased cellular activity [14, 15]. In vivo, LIUS has been shown to improve re-ossification of slow-healing adult calvarial bone [9]. LIUS seems to have several mechanisms by which it increases mass transport, including cavitation (increases cell membrane permeability) [16], acoustic microstreaming (enhances convection) [17], and thermal warming. There is also some evidence that LIUS can have effects on gene expression and cell differentiation that might relate to direct effects on cellular mechanotransduction [18–20]. In C2C12 cells, LIUS stimulation enhanced differentiation of C2C12 into osteoblast and chondroblast lineages via ERK1/2 signaling [19]. LIUS therefore seems to be a promising modality to explore for its potential to improve adipose tissue graft viability by promoting cell survival. If it can increase the viability of adipose autograft tissue, it could potentially be applied in a noninvasive manner to autograft sites. For initial studies, we characterized the effect of LIUS on dye diffusion and temperature in tissue culture plates and then on proliferation, metabolic activities, and gene expression of cell and tissue models grown in these same plates. For cells, we used C2C12 muscle cells in 2D culture because this is a well-known cell type that responses to LIUS stimulation inducing differentiation of the cells [19, 21]. For tissue, we used lipoaspirated human abdominal adipose, which was cleaned and rendered into “organoids”. LIUS appears to increase proliferation and cellular activity. These, together with evidence for decreased expression of a marker for cell injury (tumor necrosis factor-alpha, TNF-α) after LIUS, suggest that LIUS may have a role in promoting adipose autograft survival.
2. Materials and Methods
2.1. Materials
Mouse muscle cell line C2C12 was purchased from ATCC (Manassas, VA). Lysonix 2000 Ultrasonic Surgical Systems were from Byron Medical Inc. (Tucson, AZ). Multiwell tissue culture plates (12-well) were from Becton Dickinson (Franklin Lakes, NJ).
2.1.1. Dye Visualization of Indirect LIUS
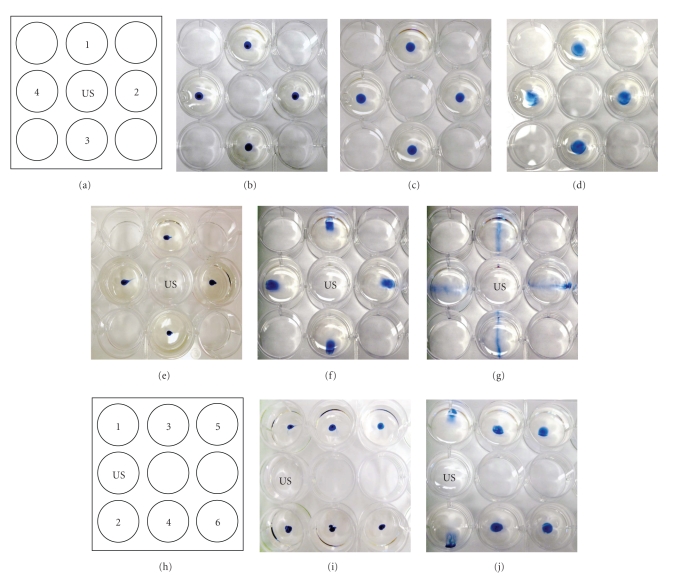
Two configurations were designed to visualize the effects of LIUS stimulation on diffusion (Figures 1(a) and 1(h)). Two microliters of trypan blue dye were loaded in the center of wells containing 3 mL of DMEM media (Figures 1(b), 1(e), and 1(i)). For controls, the wells were left for 3 or 10 minutes without treatment (Figures 1(c) and 1(d)). For stimulated groups, an ultrasound probe was placed 2 mm above the bottom of the indicated well (Figures 1(a) and 1(h)), and 22 kHz LIUS at a power level of 30 mW/cm2 (level 3 on unit) was applied for 3 (Figures 1(f) and 1(j) or 10 minutes (Figure 1(g)). Diffusion patterns were documented photographically.
Figure 1.
Dye visualization of indirect LIUS. (a) Schematic of indirect LIUS setup. Ultrasound probe was located in the US well. In controls, dye was loaded in wells and incubated for 0 minute (b), 3 minutes (c), or 10 minutes (d) without stimulation. For the stimulated group, dye was incubated for 0 minute (e), 3 minutes (f), or 10 minutes (g) with indirect LIUS. (h) Schematic of indirect LIUS setup for adipose graft stimulation. Dye was loaded in wells and incubated for 0 minute (i) or 3 minutes (j) with indirect LIUS (N = 3). With LIUS stimulation, dispersion of dye was visible in 3 minutes.
2.1.2. Hydrophone Calibration of Indirect LIUS
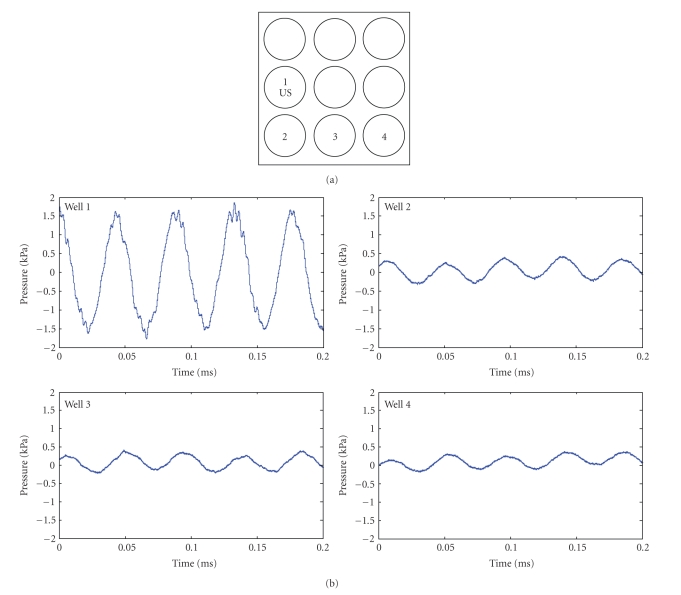
Acoustic pressure was measured using a miniature high sensitivity [−211 dB re 1 V/μPa] hydrophone (Type 8103, Brüel & Kjaer sound and vibration measurement, Denmark) calibrated over the frequency range from 0.1 Hz to 180 kHz. The high-impedance hydrophone signal was routed through a signal-conditioning charge preamplifier (Model 2525, Brüel & Kjaer, Denmark) using 30 dB of gain. Voltage at the preamplifier output was sampled with a PC-based oscilloscope (PicoScope 4224, Pico Technology, UK). Acoustic measurements were carried out using the same setup as described for the dye visualization experiments. Wells of the plate were filled with 3 mL of deionized water. The emitting US transducer was mounted vertically facing the center of well 1 (Figure 2(a)), and the hydrophone was immersed centrally in wells 2, 3, and 4. To allow for background correction, the signal acquisition for each well was carried out twice: with and without ultrasonic excitation. Background correction was performed using Matlab (Mathworks, Natick, MA). Pressure (P) was calculated from acquired voltage readings (e) based on the manufacturer-provided charge sensitivity of the hydrophone (S = 92 × 10−3 pC/Pa) and the output voltage sensitivity of the preamplifier (Vu = 30 mV/pC) as
Figure 2.
Acoustic pressure of the indirect LIUS. (a) Schematic of indirect LIUS setup. Well 1 had the highest pressure and well 4 had the lowest pressure measured.
| (1) |
2.1.3. C2C12 Cell Culture and Indirect LIUS Stimulation
C2C12 cells were seeded at 50,000 cells per well and incubated for 24 hours to permit cell attachment to the plate. On day 2, LIUS at 30 mW/cm2 was applied to a central well, as shown in Figure 1(a), for 3 minutes or 10 minutes per day for 6 days. This regimen was chosen based on pilot experiments showing that the effects of LIUS on cell permeability are quite persistent, with changes lasting as long as 6 days. The stimulations were conducted at room temperature in a sterile hood with the culture plate firmly taped to the bench surface and then the cultures were returned to a standard 37°C CO2 incubator. Control plates were treated identically but no LIUS was applied.
2.1.4. Adipose Tissue Isolation and Indirect LIUS Stimulation
All procedures involving human tissues were followed by the MGH guideline for human subject handling. Human abdominoplasty specimens (N = 2) were obtained from 20–30 year-old female donors by excision and were then further processed by lipo-aspiration and washed with saline to collect smaller sized pieces of (~2–5 mm) adipose tissue, which we term organoids [22]. The whole process took 4-5 hours and the samples were kept at 4°C during such time. The adipose organoids were then placed in 12-well tissue culture plates (500 mg of tissue with 3 mL of culture media). Indirect LIUS was applied by placing the probe in culture medium in a middle well (Figure 1(h), marked as US), while the tissue samples were placed in the outer wells (Figure 1(h)). For 6 days, LIUS stimulation was applied for 3 minutes with a power level setting of 30 mW/cm2. In the control plates, no LIUS was applied.
2.2. Assays
2.2.1. Cell Counting
Cell growth was measured by counting cells using a hemocytometer (VWR, Bridgeport, NJ). Two independent experiments (N = 2) with each in quadruplicate were performed (N = 4) (total N = 8).
2.2.2. Metabolic Measurement
Glucose consumption and lactate production were measured on days 2, 4, and 6 by analyzing the culture media with Stat Critical Care Xpress analyzer (Nova Biomedical, Waltham, MA). This assay measures the remaining glucose and production of lactate in the culture medium. Fresh culture medium was used as the baseline condition.
2.2.3. Proliferation Assay
Total DNA content was measured using the PicoGreen assay (Molecular Probes, Eugene, OR). The adipose tissue was suspended in an extraction buffer (1 N NH4OH, 0.2% Triton X-100) and treated with a bead beater (BioSpec Products, Bartlesville, OK). Next, the samples were centrifuged at 10,000 ×g for 10 minutes to remove debris, and the extract was used for the PicoGreen assay following the manufacturer's instruction.
2.2.4. Viability Assay
Cell viability was measured by reduction of AlamarBlue, a colorimetric indicator of reduction/oxidation. The cultured adipose organoids (500 mg) were incubated in 2 ml culture media and 10% (v/v) of AlamarBlue solution was added. The rate of reduction/oxidation of AlamarBlue was measured at 0, 2, 4, 6, 12, and 24 hours. At each time point, the absorbance of the culture medium was measured at 570 and 600 nm. The metabolic rate was calculated by an equation provided by the manufacturer using the measured absorbance.
2.3. Real-Time PCR
Total RNA from the adipose specimens was isolated using the RNeasy Mini kit (Qiagen, CA). A two-step real-time PCR reaction was performed. Briefly, 1 μg of total RNA was incubated with oligo-dT and reverse transcription was performed. After the reverse transcription, 200 ng of the cDNA were used for real-time PCR using TaqMan Gene expression assay systems (Applied Biosystems, Foster City, CA). Taqman Universal PCR mix, TNF-α and GAPDH primers (Assays-on-demand products) and cDNA were mixed, and the PCR reaction was performed at 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Expression of the TNF-α gene was measured to assess cellular damage during LIUS stimulation. GAPDH was used as an endogenous control and the average cycle threshold (Ct) of 3 replicates was used for the calculation. The fold difference of target genes was determined relative to the GAPDH gene (Relative expression = 2[−(Ctsample − CtGAPDH)]).
2.4. Histology
Adipose organoids were fixed in 10% formalin for 24 hours, dehydrated with a graded series of ethanol, embedded in paraffin, and then sectioned at 5 μm thickness. The sections were stained with Masson's trichrome for histological analysis.
2.4.1. Immunohistochemistry
The C2C12 cells were fixed in 10% formalin for 2 hours and permeabilized with 0.1% Triton-X100 for 30 minutes to increase antibody binding. The cells were incubated with 10% horse serum for 40 minutes at room temperature and incubated for 1 hour at 37°C with antitropomyosin (Sigma, St. Louis, MO) at a concentration of 1 : 100 diluted in PBS containing 0.5% Tween 20 and 1.5% horse serum. Fluorescence-conjugated secondary antibodies (Fluorescence conjugated-anti-mouse IgG, Vector Laboratories, CA, 1 : 200 dilution) were used. A fluorescence microscope (Nikon Eclipse E600, Japan) was used to observe sections.
2.5. Statistical Analysis
Statistical analysis was performed using single factor ANOVA with Scheffe post-hoc test (P < .05).
3. Results
3.1. Dye Diffusion
LIUS enhanced the movement of dye (compared to controls) in wells adjacent to the LIUS probe for the two plate configurations shown in Figures 1(a) and 1(h). In both instances, the dye in the wells adjacent to the LIUS moved away from the LIUS probe. After 3 minutes the entire aliquot of dye appeared to have translocated (Figures 1(f) and 1(j)), while after 10 minutes the dye was more dispersed throughout the well, with the most concentrated dye in a radial orientation relative to the LIUS well (Figure 1(g)). In the configuration where some wells were not adjacent to the LIUS probe (Figure 1(h), wells 3, 4, 5 & 6), there was less effect of the LIUS on movement and diffusion of the dye.
3.2. Characterization of Indirect LIUS by Hydrophone
A hydrophone was used to measure the acoustic pressure generated by the ultrasound probe and examples of the recorded acoustic waveforms are shown in Figure 2. The well containing the LIUS probe (well 1) had an acoustic pressure of 1500 Pa. The wells 2, 3, and 4 showed significant attenuation of the acoustic pressure relative to the well containing the probe. Pressure in those wells was correlated with their distance from the probe (well 2: 341 Pa, well 3: 228 Pa, and well 4: 150 Pa).
3.3. Effects on C2C12 Cells
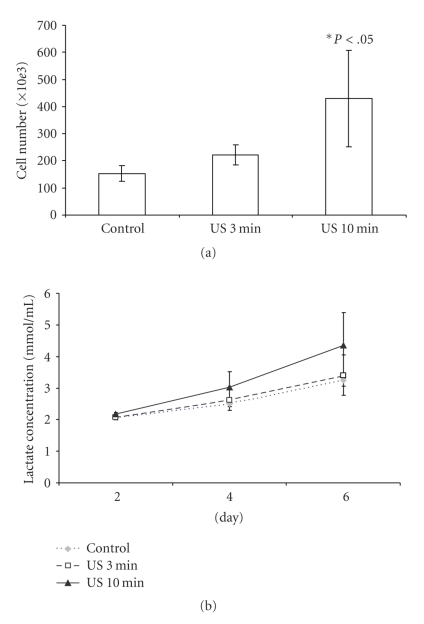
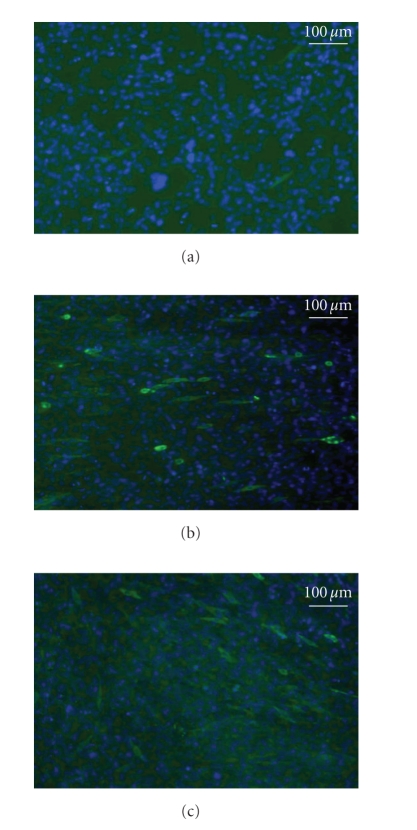
C2C12 cells grown with LIUS stimulation showed significantly increased proliferation (Figure 3(a)) in a dose-dependent manner. Following 6 days of daily stimulation, the 10 minutes group showed a twofold increase in total cell number to control (P < .05). Cells with LIUS stimulation also showed higher lactate production at days 4 and 6 compared to controls (Figure 3(b)). C2C12 cells stimulated with LIUS showed positive staining for tropomyosin (a muscle protein) but controls were negative for the stain (Figure 4). Although the stimulated group had higher expression of tropomyosin than the nonstimulated groups, there was no significant difference in expression of tropomyosin in the 3 or 10 minutes stimulated samples.
Figure 3.
Proliferation and metabolic activity of C2C12 cells stimulated with indirect LIUS. (a) C2C12 cells were stimulated for 3 or 10 minutes daily for 6 days and total cell number was measured by trypan blue exclusion methods on day 6. The control was not stimulated (P < .05, N = 8). (b) Lactate concentration in the culture media was measured at days 2, 4, and 6 (N = 4). LIUS stimulation increased cell proliferation and metabolic activity of C2C12 cells.
Figure 4.
Immunofluorescence of tropomyosin. C2C12 cells were cultured for 6 days without stimulation (a) or with daily stimulation for 3 minutes (b) or 10 minutes (c). LIUS induced tropomyosin expression in both 3- and 10-minute stimulated groups. Tropomyosin positive cells stained green and nuclei stained blue.
3.4. Effects on Adipose Organoids
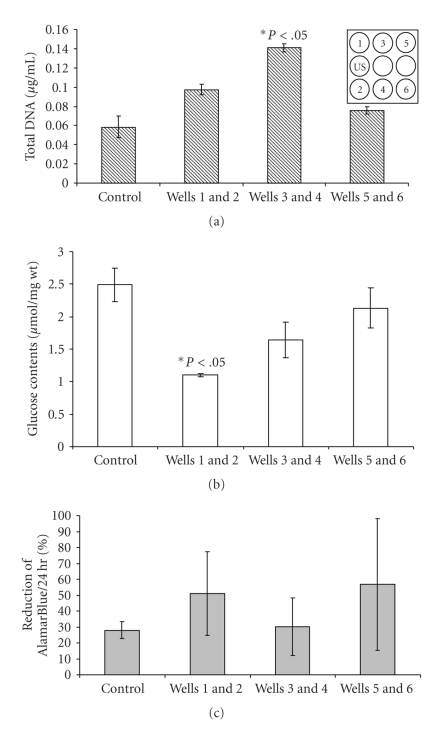
Following 6 days of daily LIUS stimulation for 3 minutes, total DNA assays of the adipose organoids showed significantly higher growth in the wells 3 and 4 compared to the control (Wells 3 and 4, P < .05). Wells 3 and 4 had mean DNA levels that were double of that of the controls. Wells 5 and 6 did not differ from controls (Figure 5(a)). Glucose levels in the culture media of wells 1 and 2 were reduced following LIUS, indicating enhanced glucose uptake and thus increased metabolic activity compared to the control (Figure 5(b), P < .05). Cell viability tested by AlamarBlue reduction showed similar viability in all samples (Figure 5(c)).
Figure 5.
Measures of proliferation, metabolic activity and viability. Adipose organoids were cultured with indirect LIUS for 3 minutes daily for 6 days. (a) Total DNA of the adipose organoid was measured on day 6 by PicoGreen assay (P < .05, N = 3). (b) Glucose from the culture medium was measured after 6 days of culture (P < .05, N = 3). (c) Viability of the cultured adipose organoid was measured by AlamarBlue reduction over 24 hours on day 6 (N = 3). Insert shows schematic of LIUS setup. LIUS enhanced adipose organoid proliferation and metabolic activity, but not viability.
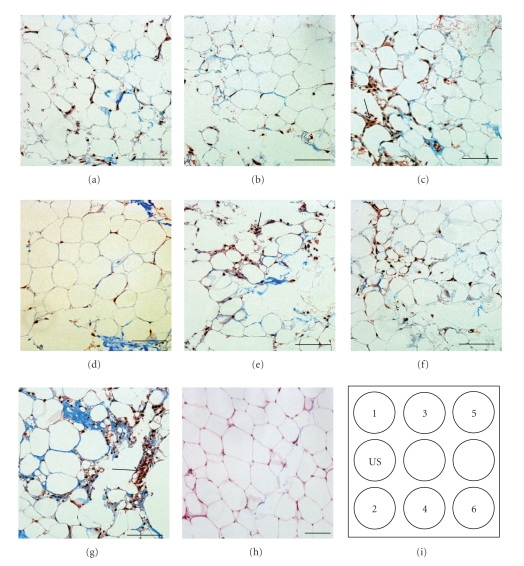
Several morphological effects on adipocytes were observed in the organoids stained with trichrome. First, in the nonstimulated group and in samples from wells 5 and 6, there appeared to be damaged adipocyte profiles and smaller diameter adipocytes (Figures 6(e)–6(g)). There was also an apparent increase in the fraction of stromal cells relative to the number of mature adipocytes (also seen for well 3, Figure 6(c) and normal tissue Figure 6(h)). The shape of the mature adipocytes appeared to be best preserved for organoids from wells 1, 2, and 4.
Figure 6.
Histology of the adipose organoids. The adipose organoids were cultured for 6 days with indirect LIUS stimulation for 3 minutes daily. The adipose tissue as from wells 1 (a), 2 (b), 3 (c), 4 (d), 5 (e), 6 (f), and control (g) were stained with Masson's trichrome to visualize collagen fibers (blue) and cells (red). (h) Histology of normal adipose tissue was shown as standard morphology. Schematic of LIUS setup was shown in (i). Arrows indicate stromal cells. Scale bars, 200 μm. The adipocytes from wells 1, 2, and 4 had morphology similar to normal tissue (h).
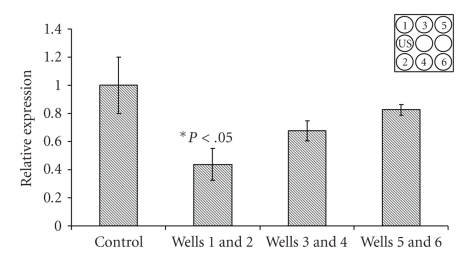
Expression of the TNF-α gene in the LIUS stimulated group was lower than control for wells 1 and 2 (Figure 7, P < .05).
Figure 7.
Relative mRNA expression of TNF-α in the adipose organoids. The organoids were cultured for 6 days with indirect LIUS for 3 min daily. Insert shows schematic of LIUS setup (P < .05, N = 4). Wells 1 and 2 showed about 2-fold lower expression of TNF-α compared to the control.
4. Discussion
4.1. Dye Visualization and Acoustic Pressure Measurement
Dye spread data indicated that LIUS enhances mass transport of dye molecules (in this case trypan blue, which has a molecular weight of 961 Da). After 10 minutes of LIUS, increased transport of the dye was observed, producing a remarkable radial dispersion (Figure 1(g)) in wells adjacent to the LIUS probe. It is likely that the radial pattern is the result of reflection of the LIUS energy from the walls of the plastic tissue culture plate. These effects are probably highly dependent on the geometry of the setup and are not expected to appear in the ultimate clinical use of LIUS; however, they do serve as a reminder that the local levels of the ultrasound are influenced by local variations in tissue acoustic impedances [23]. It also indicates that the LIUS intensity experienced by samples in the test wells was not uniform, though it does appear to have been affected as a function of proximity to the probe.
Acoustic pressure measurement using hydrophone clearly demonstrated that wells adjacent to the probe received more LIUS stimulation than the wells located far away from the probe (Figure 2). This data suggests that depending on location of the probe one can expect variable effects of LIUS, which eventually may be possible to manipulate cellular responses.
4.2. Effects of LIUS on Cells in 2D Culture
In conventional 2D cell culture systems, culture medium is typically refreshed every 2-3 days; therefore, it is believed that nutrient and gas supply are not limiting factors for cell growth. It is not clear whether the increased metabolic activity, proliferation, and increased tropomyosin expression of LIUS-stimulated C2C12 cells can be explained by increased mass transport, since this is not thought to be a limiting factor for 2D cell culture. Another possibility is that mechanotransduction signaling in response to LIUS-induced shear stress could have stimulated changes in the C2C12 cells, similar to the mechanism proposed in LIUS experiments with chondrocytes [11]. For example, Zhou et al. [24] demonstrated that mechanotransduction of acoustic pulsed energy of ultrasound by skin fibroblasts is mediated by integrin receptors and the ERK 1/2 and RhoA signaling pathways. In the context of using LIUS to enhance autograft survival, the possibility that the LIUS can directly activate signaling pathways in implanted cells needs to be taken into account. Tropomyosin expression was higher in the LIUS-stimulated groups than the controls, suggesting that LIUS stimulates differentiation of C2C12 cells under these conditions. Both the 3- and the 10-minute stimulated groups showed similar expression of tropomyosin, perhaps because longer exposure may predominantly stimulate cell proliferation rather than cell differentiation.
4.3. Effects of LIUS on Adipose Organoids
The enhanced proliferation and uptake of glucose by the adipose organoids, as well as some indications of intact tissue morphology and reduced TNF-α expression, are suggestive of improved permeation of oxygen and nutrients into the organoids. The size of the organoids ranged from 2–5 mm in diameter and thus the interior of cells is beyond the distance of 0.2 mm that is deemed sufficient for diffusion of molecules in a tissue culture situation [25, 26]. In this study, we evaluated a single dose of LIUS on the organoids: 3 minutes of stimulation, applied once daily at an intensity of 30 mW/cm2 for 6 days following previous studies (data not shown). Obviously there are many parameters that can be adjusted, and further study is needed to optimize intensity, frequency, and duty cycle of the LIUS depending on cell and tissue types. The in vitro model presented here may be useful for evaluating some of these parameters prior to in vivo studies.
TNF-α was used as an indicator of tissue damage, since release of this cytokine is typically associated with tissue injury [27]. But TNF-α also modulates other cellular function such as recruiting mesenchymal stem cells in myocardial infarction [28] and induction of apoptosis in chondrocytes [29]. The LIUS-related reduction in TNF-α secretion seems to indicate that the LIUS had a beneficial effect on organoid survival. Young and Dyson [30] reported that US-treated tissue contains less macrophages (meaning less TNF-α) than control. However, it has also been reported that LIUS stimulation of bone can increase TNF-α levels [31] participating in fracture healing. This discrepancy may be due to different stimulation settings (indirect versus direct stimulation), different stimulation intensity (22 kHz, 1 MHz, 3 MHz), or different cell and tissue types (adipose tissue versus bone).
LIUS has been used to enhance bone healing, repair of damaged muscle, and differentiation of mesenchymal stem cells into osteoblasts or chondrocytes [19, 32, 33]. It will be important to test for in vivo effects of LIUS on damaged soft tissue to assess whether our in vitro results can be confirmed in vivo. Nonthermal effects such as microbubbles and microstreaming generated by LIUS can enhance ionic transport, permeability of the cell membrane and cellular activity [16, 34]. In our study, upon LIUS stimulation, C2C12 muscle cells clearly showed higher cell proliferation and differentiation than the nonstimulated groups. In addition, we obtained preliminary evidence that LIUS can influence the viability of adipose organoids in an in vitro organ culture model.
5. Conclusion
Our data suggests that indirect LIUS stimulation may enhance C2C12 cell proliferation, metabolic activity, and differentiation of cells and tissues. It may be possible to implement LIUS stimulation to increase survival of implanted soft tissue.
Acknowledgments
Authors thank William G. Austen, M.D. from the Division of Plastic Surgery at Massachusetts General Hospital for his assistance with this investigation and Alexander Augst, Ph.D. for his help with statistical analyses. This research was supported by the Institute of Laryngology and Voice Restoration and the Eugene B. Casey Foundation. This work was sponsored by NIH grant #2R01EB000351-17A1, and the U.S. Army Research Office through the Institute for Soldier Nanotechnologies at MIT under Contract W911NF-07-D-0004 ISN Project 2.3.2: “Non-Invasive Delivery and Sensing” and ISN Project 4.1.2: “Switchable Surfaces and Novel Elastomers for Improving Cell Function and Device Performance of Cell-based Biosensors,” and the content of the information does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred.
References
- 1.Duke SG, Salmon J, Blalock PD, Postma GN, Koufman JA. Fascia augmentation of the vocal fold: graft yield in the canine and preliminary clinical experience. Laryngoscope. 2001;111(5):759–764. doi: 10.1097/00005537-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Sommer B, Sattler G. Current concepts of fat graft survival: histology of aspirated adipose tissue and review of the literature. Dermatologic Surgery. 2000;26(12):1159–1166. [PubMed] [Google Scholar]
- 3.Neuber F. Fat transplantation. Chirurgische Kongress der Deutschen Gesellschaft für Chirurgie Verh. 1893;22:p. 66. [Google Scholar]
- 4.Shaw GY, Szewczyk MA, Searle J, Woodroof J. Autologous fat injection into the vocal folds: technical considerations and long-term follow-up. Laryngoscope. 1997;107(2):177–186. doi: 10.1097/00005537-199702000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Ersek RA. Transplantation of purified autologous fat: a 3-year follow-up is disappointing. Plastic and Reconstructive Surgery. 1991;87(2):219–228. [PubMed] [Google Scholar]
- 6.Ford CN, Staskowski PA, Bless DM. Autologous collagen vocal fold injection: a preliminary clinical study. Laryngoscope. 1995;105(9):944–948. doi: 10.1288/00005537-199509000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Brandenburg JH, Unger JM, Koschkee D. Vocal cord injection with autogenous fat: a long-term magnetic resonance imaging evaluation. Laryngoscope. 1996;106(2):174–180. doi: 10.1097/00005537-199602000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Klein AW, Elson ML. The history of substances for soft tissue augmentation. Dermatologic Surgery. 2000;26(12):1096–1105. [PubMed] [Google Scholar]
- 9.Gleizal A, Li S, Pialat J-B, Beziat J-L. Transcriptional expression of calvarial bone after treatment with low-intensity ultrasound: an in vitro study. Ultrasound in Medicine & Biology. 2006;32(10):1569–1574. doi: 10.1016/j.ultrasmedbio.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Harvey W, Dyson M, Pond JB, Grahame R. The stimulation of protein synthesis in human fibroblasts by therapeutic ultrasound. Rheumatology and Rehabilitation. 1975;14(4):p. 237. doi: 10.1093/rheumatology/14.4.237. [DOI] [PubMed] [Google Scholar]
- 11.Noriega S, Mamedov T, Turner JA, Subramanian A. Intermittent applications of continuous ultrasound on the viability, proliferation, morphology, and matrix production of chondrocytes in 3D matrices. Tissue Engineering. 2007;13(3):611–618. doi: 10.1089/ten.2006.0130. [DOI] [PubMed] [Google Scholar]
- 12.Dyson M, Luke DA. Induction of mast cell degranulation in skin by ultrasound. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 1986;33(2):194–201. doi: 10.1109/t-uffc.1986.26814. [DOI] [PubMed] [Google Scholar]
- 13.Wells PNT. Ultrasonics in medicine and biology. Physics in Medicine and Biology. 1977;22(4):629–669. doi: 10.1088/0031-9155/22/4/001. [DOI] [PubMed] [Google Scholar]
- 14.Park K, Hoffmeister B, Han DK, Hasty K. Therapeutic ultrasound effects on interleukin-1β stimulated cartilage construct in vitro. Ultrasound in Medicine & Biology. 2007;33(2):286–295. doi: 10.1016/j.ultrasmedbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Takayama T, Suzuki N, Ikeda K, et al. Low-intensity pulsed ultrasound stimulates osteogenic differentiation in ROS 17/2.8 cells. Life Sciences. 2007;80(10):965–971. doi: 10.1016/j.lfs.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 16.Tran TA, Roger S, Le Guennec JY, Tranquart F, Bouakaz A. Effect of ultrasound-activated microbubbles on the cell electrophysiological properties. Ultrasound in Medicine & Biology. 2007;33(1):158–163. doi: 10.1016/j.ultrasmedbio.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Dyson M, Suckling J. Stimulation of tissue repair by ultrasound: a survey of the mechanisms involved. Physiotherapy. 1978;64(4):105–108. [PubMed] [Google Scholar]
- 18.Lee HJ, Choi BH, Min B-H, Son YS, Park SR. Low-intensity ultrasound stimulation enhances chondrogenic differentiation in alginate culture of mesenchymal stem cells. Artificial Organs. 2006;30(9):707–715. doi: 10.1111/j.1525-1594.2006.00288.x. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda K, Takayama T, Suzuki N, Shimada K, Otsuka K, Ito K. Effects of low-intensity pulsed ultrasound on the differentiation of C2C12 cells. Life Sciences. 2006;79(20):1936–1943. doi: 10.1016/j.lfs.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 20.Cui JH, Park K, Park SR, Min B-H. Effects of low-intensity ultrasound on chondrogenic differentiation of mesenchymal stem cells embedded in polyglycolic acid: an in vivo study. Tissue Engineering. 2006;12(1):75–82. doi: 10.1089/ten.2006.12.75. [DOI] [PubMed] [Google Scholar]
- 21.Allen RE, Stromer MH, Goll DE, Robson RM. Synthesis of tropomyosin in cultures of differentiating muscle cells. The Journal of Cell Biology. 1978;76(1):98–104. doi: 10.1083/jcb.76.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park H, Williams R, Goldman N, et al. Comparison of effects of 2 harvesting methods on fat autograft. Laryngoscope. 2008;118(8):1493–1499. doi: 10.1097/MLG.0b013e3181735634. [DOI] [PubMed] [Google Scholar]
- 23.Speed CA. Therapeutic ultrasound in soft tissue lesions. Rheumatology. 2001;40(12):1331–1336. doi: 10.1093/rheumatology/40.12.1331. [DOI] [PubMed] [Google Scholar]
- 24.Zhou S, Schmelz A, Seufferlein T, Li Y, Zhao J, Bachem MG. Molecular mechanisms of low intensity pulsed ultrasound in human skin fibroblasts. Journal of Biological Chemistry. 2004;279(52):54463–54469. doi: 10.1074/jbc.M404786200. [DOI] [PubMed] [Google Scholar]
- 25.Bursac N, Papadaki M, Cohen RJ, et al. Cardiac muscle tissue engineering: toward an in vitro model for electrophysiological studies. American Journal of Physiology. 1999;277(2):H433–H444. doi: 10.1152/ajpheart.1999.277.2.H433. [DOI] [PubMed] [Google Scholar]
- 26.Radisic M, Deen W, Langer R, Vunjak-Novakovic G. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. American Journal of Physiology. 2005;288(3):H1278–H1289. doi: 10.1152/ajpheart.00787.2004. [DOI] [PubMed] [Google Scholar]
- 27.Rink L, Kirchner H. Recent progress in the tumor necrosis factor-α field. International Archives of Allergy and Immunology. 1996;111(3):199–209. doi: 10.1159/000237369. [DOI] [PubMed] [Google Scholar]
- 28.Corallini F, Secchiero P, Beltrami AP, et al. TNF-α modulates the migratory response of mesenchymal stem cells to TRAIL. Cellular and Molecular Life Sciences. 2010;67(8):1307–1314. doi: 10.1007/s00018-009-0246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aizawa T, Kon T, Einhorn TA, Gerstenfeld LC. Induction of apoptosis in chondrocytes by tumor necrosis factor-alpha. Journal of Orthopaedic Research. 2001;19(5):785–796. doi: 10.1016/S0736-0266(00)00078-4. [DOI] [PubMed] [Google Scholar]
- 30.Young SR, Dyson M. Effect of therapeutic ultrasound on the healing of full-thickness excised skin lesions. Ultrasonics. 1990;28(3):175–180. doi: 10.1016/0041-624x(90)90082-y. [DOI] [PubMed] [Google Scholar]
- 31.Iwabuchi S, Ito M, Hata J, Chikanishi T, Azuma Y, Haro H. In vitro evaluation of low-intensity pulsed ultrasound in herniated disc resorption. Biomaterials. 2005;26(34):7104–7114. doi: 10.1016/j.biomaterials.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Kristiansen TK, Ryaby JP, McCabe J, Frey JJ, Roe LR. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound: a multicenter, prospective, randomized, double-blind, placebo-controlled study. The Journal of Bone and Joint Surgery. 1997;79(7):961–973. doi: 10.2106/00004623-199707000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Chia S-L, Gorna K, Gogolewski S, Alini M. Biodegradable elastomeric polyurethane membranes as chondrocyte carriers for cartilage repair. Tissue Engineering. 2006;12(7):1945–1953. doi: 10.1089/ten.2006.12.1945. [DOI] [PubMed] [Google Scholar]
- 34.Claes L, Willie B. The enhancement of bone regeneration by ultrasound. Progress in Biophysics and Molecular Biology. 2007;93(1–3):384–398. doi: 10.1016/j.pbiomolbio.2006.07.021. [DOI] [PubMed] [Google Scholar]