Figure 1.
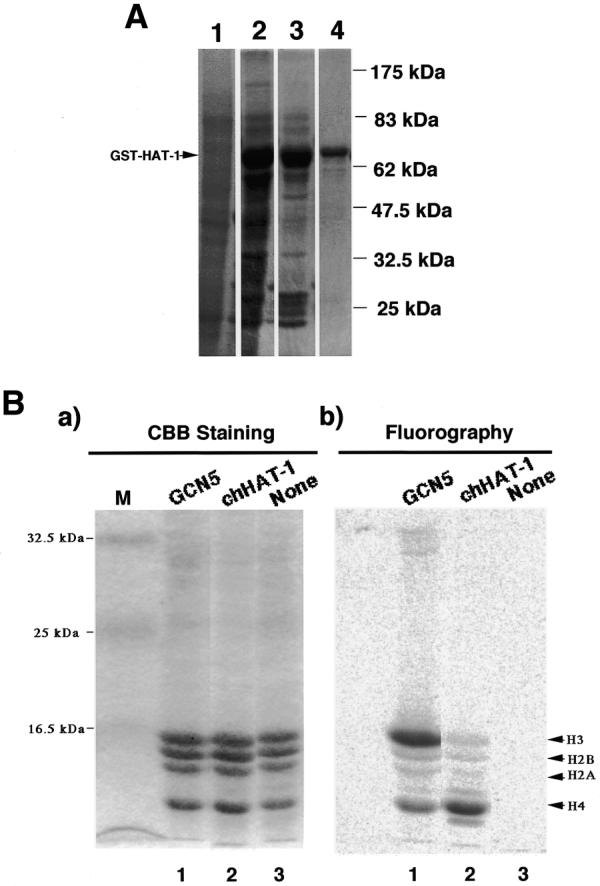
SDS–gel electrophoretic patterns of GST–chHAT-1 fusion protein-containing fractions at different purification steps, and substrate specificity of the recombinant chHAT-1. (A) Protein samples prepared were subjected to 10% SDS–PAGE, followed by Coomassie brilliant blue staining. Lane 1, whole cell lysate of BL-21 cells containing the pGEX-2TKchHAT-1 plasmid without induction by IPTG; lane 2, lysate of BL-21 cells containing the pGEX-2TKchHAT-1 plasmid with induction by 50 µM IPTG; lane 3, complex beads containing chHAT-1; lane 4, chHAT-1 fraction purified with glutathione–Sepharose beads. The standard molecular weights are indicated. GST–HAT-1, GST–chHAT-1 fusion protein. (B) The in vitro acetylation reaction mixture (described below) was subjected to 15% SDS–PAGE, fixed, stained with Coomassie brilliant blue (a) and then exposed to an imaging plate (b). Lane 1, recombinant chicken GCN5 (our unpublished data); lane 2, recombinant chHAT-1; lane 3, without the enzyme as a negative control, using chicken histones prepared from DT40 cells as substrates.
