Abstract
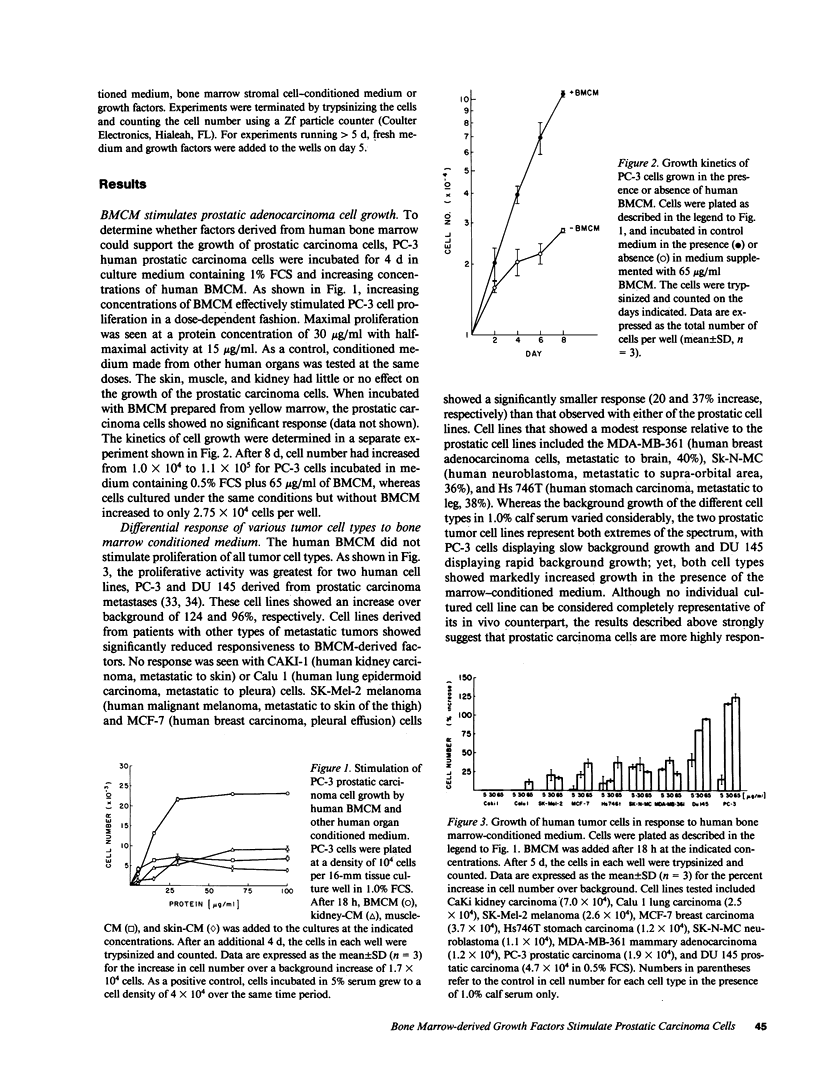
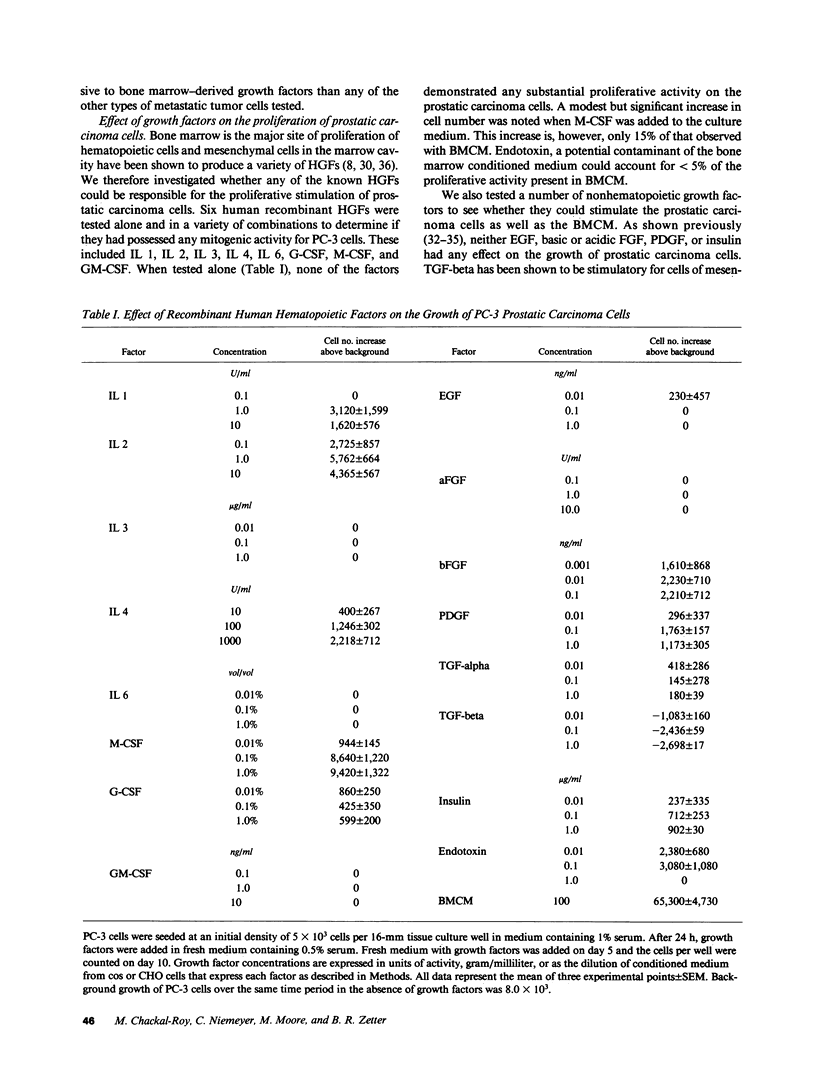
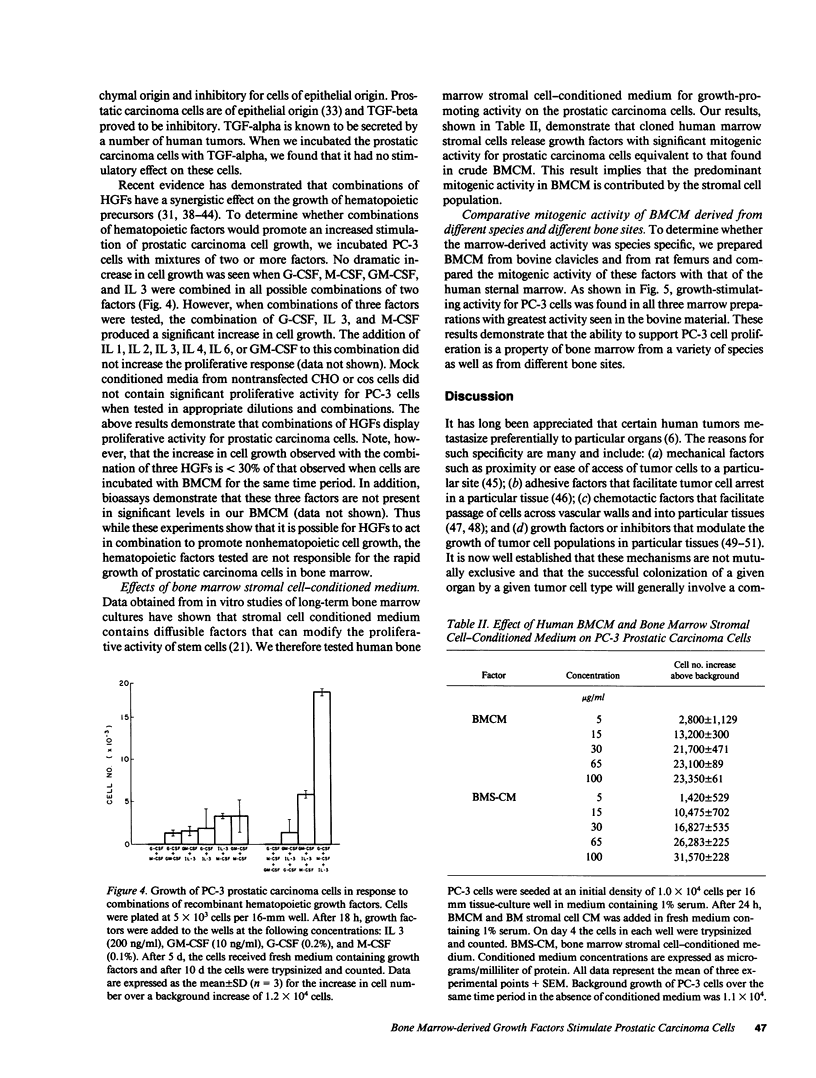
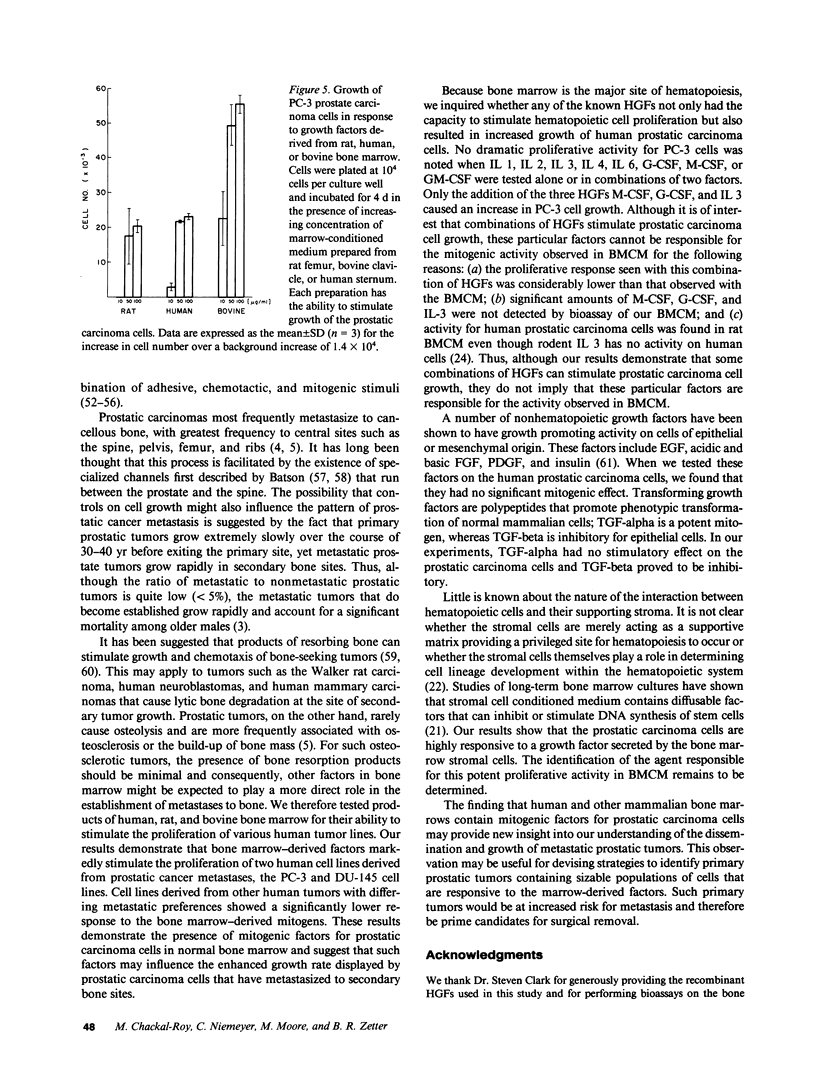
Malignant prostatic carcinoma, a major cause of cancer mortality in males, most often metastasizes to secondary sites in bone. Frequently, the growth rate of the secondary tumor in bone marrow is considerably greater than that of the slowly growing primary prostatic tumor. We now report that two lines of human prostatic carcinoma cells proliferate in response to conditioned media from unstimulated human, rat, or bovine bone marrow. Nonprostatic tumor cell lines showed little or no growth response to the same medium. The proliferative activity found in bone marrow was not duplicated by any of a variety of purified growth factors including epidermal growth factor (EGF), acidic or basic fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), transforming growth factor (TGF) alpha or beta, interleukins 1, 2, 3, 4 or 6, granulocyte (G), macrophage (M) or granulocyte-macrophage (GM) colony stimulating factor (CSF). Whereas a mixture of G-CSF, M-CSF, and IL 3 produced a mitogenic response in the prostatic carcinoma cells, these three factors were not present in our bone marrow samples in sufficient quantities to promote the observed proliferative response. To further identify the cellular source of the proliferative activity present in bone marrow-conditioned medium, we tested conditioned media made from human bone marrow stromal cells. The stromal cell conditioned medium stimulated increased growth of the prostatic carcinoma cells to levels equivalent to those observed with the bone marrow conditioned medium. These results suggest that novel mitogenic factors that are produced by bone marrow stromal cells and remain in the bone marrow cavity may account, in part, for the preferential growth of prostatic metastases in bone.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batson O. V. THE FUNCTION OF THE VERTEBRAL VEINS AND THEIR ROLE IN THE SPREAD OF METASTASES. Ann Surg. 1940 Jul;112(1):138–149. doi: 10.1097/00000658-194007000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettoni B. A., Carter J. R. Mechanisms of cancer metastasis to bone. J Bone Joint Surg Am. 1986 Feb;68(2):308–312. [PubMed] [Google Scholar]
- Broudy V. C., Zuckerman K. S., Jetmalani S., Fitchen J. H., Bagby G. C., Jr Monocytes stimulate fibroblastoid bone marrow stromal cells to produce multilineage hematopoietic growth factors. Blood. 1986 Aug;68(2):530–534. [PubMed] [Google Scholar]
- Broxmeyer H. E. Biomolecule-cell interactions and the regulation of myelopoiesis. Int J Cell Cloning. 1986 Nov;4(6):378–405. doi: 10.1002/stem.5530040601. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., Williams D. E., Hangoc G., Cooper S., Gillis S., Shadduck R. K., Bicknell D. C. Synergistic myelopoietic actions in vivo after administration to mice of combinations of purified natural murine colony-stimulating factor 1, recombinant murine interleukin 3, and recombinant murine granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3871–3875. doi: 10.1073/pnas.84.11.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B. D., Clark C. R. Interleukin 3 (IL 3) regulates the in vitro proliferation of both blood monocytes and peritoneal exudate macrophages: synergism between a macrophage lineage-specific colony-stimulating factor (CSF-1) and IL 3. J Immunol. 1986 Jul 15;137(2):563–570. [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Dexter T. M. Stromal cell associated haemopoiesis. J Cell Physiol Suppl. 1982;1:87–94. doi: 10.1002/jcp.1041130414. [DOI] [PubMed] [Google Scholar]
- Fibbe W. E., van Damme J., Billiau A., Goselink H. M., Voogt P. J., van Eeden G., Ralph P., Altrock B. W., Falkenburg J. H. Interleukin 1 induces human marrow stromal cells in long-term culture to produce granulocyte colony-stimulating factor and macrophage colony-stimulating factor. Blood. 1988 Feb;71(2):430–435. [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Gordon M. Y., Riley G. P., Watt S. M., Greaves M. F. Compartmentalization of a haematopoietic growth factor (GM-CSF) by glycosaminoglycans in the bone marrow microenvironment. 1987 Mar 26-Apr 1Nature. 326(6111):403–405. doi: 10.1038/326403a0. [DOI] [PubMed] [Google Scholar]
- Gualtieri R. J., Shadduck R. K., Baker D. G., Quesenberry P. J. Hematopoietic regulatory factors produced in long-term murine bone marrow cultures and the effect of in vitro irradiation. Blood. 1984 Aug;64(2):516–525. [PubMed] [Google Scholar]
- Hart I. R. 'Seed and soil' revisited: mechanisms of site-specific metastasis. Cancer Metastasis Rev. 1982;1(1):5–16. doi: 10.1007/BF00049477. [DOI] [PubMed] [Google Scholar]
- Hines D. L. Lipid accumulation and production of colony-stimulating activity by the 266AD cell line derived from mouse bone marrow. Blood. 1983 Feb;61(2):397–402. [PubMed] [Google Scholar]
- Horak E., Darling D. L., Tarin D. Analysis of organ-specific effects on metastatic tumor formation by studies in vitro. J Natl Cancer Inst. 1986 May;76(5):913–922. [PubMed] [Google Scholar]
- Huben R. P., Murphy G. P. Prostate cancer: an update. CA Cancer J Clin. 1986 Sep-Oct;36(5):274–292. doi: 10.3322/canjclin.36.5.274. [DOI] [PubMed] [Google Scholar]
- Hujanen E. S., Terranova V. P. Migration of tumor cells to organ-derived chemoattractants. Cancer Res. 1985 Aug;45(8):3517–3521. [PubMed] [Google Scholar]
- Hunt P., Robertson D., Weiss D., Rennick D., Lee F., Witte O. N. A single bone marrow-derived stromal cell type supports the in vitro growth of early lymphoid and myeloid cells. Cell. 1987 Mar 27;48(6):997–1007. doi: 10.1016/0092-8674(87)90708-2. [DOI] [PubMed] [Google Scholar]
- Hunt P., Robertson D., Weiss D., Rennick D., Lee F., Witte O. N. A single bone marrow-derived stromal cell type supports the in vitro growth of early lymphoid and myeloid cells. Cell. 1987 Mar 27;48(6):997–1007. doi: 10.1016/0092-8674(87)90708-2. [DOI] [PubMed] [Google Scholar]
- Jacobs S. C. Spread of prostatic cancer to bone. Urology. 1983 Apr;21(4):337–344. doi: 10.1016/0090-4295(83)90147-4. [DOI] [PubMed] [Google Scholar]
- Kaighn M. E., Narayan K. S., Ohnuki Y., Lechner J. F., Jones L. W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol. 1979 Jul;17(1):16–23. [PubMed] [Google Scholar]
- Kawasaki E. S., Ladner M. B., Wang A. M., Van Arsdell J., Warren M. K., Coyne M. Y., Schweickart V. L., Lee M. T., Wilson K. J., Boosman A. Molecular cloning of a complementary DNA encoding human macrophage-specific colony-stimulating factor (CSF-1). Science. 1985 Oct 18;230(4723):291–296. doi: 10.1126/science.2996129. [DOI] [PubMed] [Google Scholar]
- Koike K., Stanley E. R., Ihle J. N., Ogawa M. Macrophage colony formation supported by purified CSF-1 and/or interleukin 3 in serum-free culture: evidence for hierarchical difference in macrophage colony-forming cells. Blood. 1986 Apr;67(4):859–864. [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Wewer U. M. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- Lipschitz D. A., Udupa K. B., Taylor J. M., Shadduck R. K., Waheed A. Role of colony-stimulating factor in myelopoiesis in murine long-term bone marrow cultures. Blood. 1987 Apr;69(4):1211–1217. [PubMed] [Google Scholar]
- Magro C., Orr F. W., Manishen W. J., Sivananthan K., Mokashi S. S. Adhesion, chemotaxis, and aggregation of Walker carcinosarcoma cells in response to products of resorbing bone. J Natl Cancer Inst. 1985 Apr;74(4):829–838. [PubMed] [Google Scholar]
- Manishen W. J., Sivananthan K., Orr F. W. Resorbing bone stimulates tumor cell growth. A role for the host microenvironment in bone metastasis. Am J Pathol. 1986 Apr;123(1):39–45. [PMC free article] [PubMed] [Google Scholar]
- McNeal J. E., Bostwick D. G., Kindrachuk R. A., Redwine E. A., Freiha F. S., Stamey T. A. Patterns of progression in prostate cancer. Lancet. 1986 Jan 11;1(8472):60–63. doi: 10.1016/s0140-6736(86)90715-4. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986 Feb;67(2):257–267. [PubMed] [Google Scholar]
- Moore M. A., Warren D. J. Synergy of interleukin 1 and granulocyte colony-stimulating factor: in vivo stimulation of stem-cell recovery and hematopoietic regeneration following 5-fluorouracil treatment of mice. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7134–7138. doi: 10.1073/pnas.84.20.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao T., Yamauchi K., Shimizu M., Noguchi K. Regulatory role of human bone marrow fibroblasts in proliferation by granulocyte and macrophage colony-forming cells. Exp Hematol. 1986 Aug;14(7):696–701. [PubMed] [Google Scholar]
- Nagata S., Tsuchiya M., Asano S., Kaziro Y., Yamazaki T., Yamamoto O., Hirata Y., Kubota N., Oheda M., Nomura H. Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor. 1986 Jan 30-Feb 5Nature. 319(6052):415–418. doi: 10.1038/319415a0. [DOI] [PubMed] [Google Scholar]
- Naito S., Giavazzi R., Fidler I. J. Correlation between the in vitro interaction of tumor cells with an organ environment and metastatic behavior in vivo. Invasion Metastasis. 1987;7(1):16–29. [PubMed] [Google Scholar]
- Nicolson G. L. Cancer metastasis. Organ colonization and the cell-surface properties of malignant cells. Biochim Biophys Acta. 1982 Dec 21;695(2):113–176. doi: 10.1016/0304-419x(82)90020-8. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Dulski K. M. Organ specificity of metastatic tumor colonization is related to organ-selective growth properties of malignant cells. Int J Cancer. 1986 Aug 15;38(2):289–294. doi: 10.1002/ijc.2910380221. [DOI] [PubMed] [Google Scholar]
- Oblon D. J., Castro-Malaspina H., Broxmeyer H. E. The production of colony stimulating activity by monocyte enriched fractions from murine continuous bone marrow culture adherent layers. Br J Haematol. 1983 Jun;54(2):291–299. doi: 10.1111/j.1365-2141.1983.tb02098.x. [DOI] [PubMed] [Google Scholar]
- Orr F. W., Mokashi S., Delikatny J. Generation of a complement-derived chemotactic factor for tumor cells in experimentally induced peritoneal exudates and its effect on the local metastasis of circulating tumor cells. Am J Pathol. 1982 Jul;108(1):112–118. [PMC free article] [PubMed] [Google Scholar]
- Pantazis P., Pelicci P. G., Dalla-Favera R., Antoniades H. N. Synthesis and secretion of proteins resembling platelet-derived growth factor by human glioblastoma and fibrosarcoma cells in culture. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2404–2408. doi: 10.1073/pnas.82.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesenberry P., Song Z. X., McGrath E., McNiece I., Shadduck R., Waheed A., Baber G., Kleeman E., Kaiser D. Multilineage synergistic activity produced by a murine adherent marrow cell line. Blood. 1987 Mar;69(3):827–835. [PubMed] [Google Scholar]
- Rennick D., Yang G., Gemmell L., Lee F. Control of hemopoiesis by a bone marrow stromal cell clone: lipopolysaccharide- and interleukin-1-inducible production of colony-stimulating factors. Blood. 1987 Feb;69(2):682–691. [PubMed] [Google Scholar]
- Roos E. Cellular adhesion, invasion and metastasis. Biochim Biophys Acta. 1984;738(4):263–284. doi: 10.1016/0304-419x(83)90008-2. [DOI] [PubMed] [Google Scholar]
- Sachs L. The molecular control of blood cell development. Science. 1987 Dec 4;238(4832):1374–1379. doi: 10.1126/science.3317831. [DOI] [PubMed] [Google Scholar]
- Schirrmacher V. Cancer metastasis: experimental approaches, theoretical concepts, and impacts for treatment strategies. Adv Cancer Res. 1985;43:1–73. doi: 10.1016/s0065-230x(08)60942-2. [DOI] [PubMed] [Google Scholar]
- Shadduck R. K., Waheed A., Greenberger J. S., Dexter T. M. Production of colony stimulating factor in long-term bone marrow cultures. J Cell Physiol. 1983 Jan;114(1):88–92. doi: 10.1002/jcp.1041140115. [DOI] [PubMed] [Google Scholar]
- Sieff C. A., Niemeyer C. M., Nathan D. G., Ekern S. C., Bieber F. R., Yang Y. C., Wong G., Clark S. C. Stimulation of human hematopoietic colony formation by recombinant gibbon multi-colony-stimulating factor or interleukin 3. J Clin Invest. 1987 Sep;80(3):818–823. doi: 10.1172/JCI113139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg E., Lubera J. Cancer statistics, 1987. CA Cancer J Clin. 1987 Jan-Feb;37(1):2–19. doi: 10.3322/canjclin.37.1.2. [DOI] [PubMed] [Google Scholar]
- Sitaras N. M., Sariban E., Bravo M., Pantazis P., Antoniades H. N. Constitutive production of platelet-derived growth factor-like proteins by human prostate carcinoma cell lines. Cancer Res. 1988 Apr 1;48(7):1930–1935. [PubMed] [Google Scholar]
- Song Z. X., Shadduck R. K., Innes D. J., Jr, Waheed A., Quesenberry P. J. Hematopoietic factor production by a cell line (TC-1) derived from adherent murine marrow cells. Blood. 1985 Aug;66(2):273–281. [PubMed] [Google Scholar]
- Souza L. M., Boone T. C., Gabrilove J., Lai P. H., Zsebo K. M., Murdock D. C., Chazin V. R., Bruszewski J., Lu H., Chen K. K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986 Apr 4;232(4746):61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Bartocci A., Patinkin D., Rosendaal M., Bradley T. R. Regulation of very primitive, multipotent, hemopoietic cells by hemopoietin-1. Cell. 1986 Jun 6;45(5):667–674. doi: 10.1016/0092-8674(86)90781-6. [DOI] [PubMed] [Google Scholar]
- Stone K. R., Mickey D. D., Wunderli H., Mickey G. H., Paulson D. F. Isolation of a human prostate carcinoma cell line (DU 145). Int J Cancer. 1978 Mar 15;21(3):274–281. doi: 10.1002/ijc.2910210305. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Tidmarsh G. F., Muller-Sieburg C., Weissman I. L. Bone marrow stromal cell lines with lymphopoietic activity express high levels of a pre-B neoplasia-associated molecule. Cell. 1987 Mar 27;48(6):1009–1021. doi: 10.1016/0092-8674(87)90709-4. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Temple P. A., Leary A. C., Witek-Giannotti J. S., Yang Y. C., Ciarletta A. B., Chung M., Murtha P., Kriz R., Kaufman R. J. Human CSF-1: molecular cloning and expression of 4-kb cDNA encoding the human urinary protein. Science. 1987 Mar 20;235(4795):1504–1508. doi: 10.1126/science.3493529. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Ciarletta A. B., Temple P. A., Chung M. P., Kovacic S., Witek-Giannotti J. S., Leary A. C., Kriz R., Donahue R. E., Wong G. G. Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell. 1986 Oct 10;47(1):3–10. doi: 10.1016/0092-8674(86)90360-0. [DOI] [PubMed] [Google Scholar]
- Zipori D., Duksin D., Tamir M., Argaman A., Toledo J., Malik Z. Cultured mouse marrow stromal cell lines. II. Distinct subtypes differing in morphology, collagen types, myelopoietic factors, and leukemic cell growth modulating activities. J Cell Physiol. 1985 Jan;122(1):81–90. doi: 10.1002/jcp.1041220113. [DOI] [PubMed] [Google Scholar]


