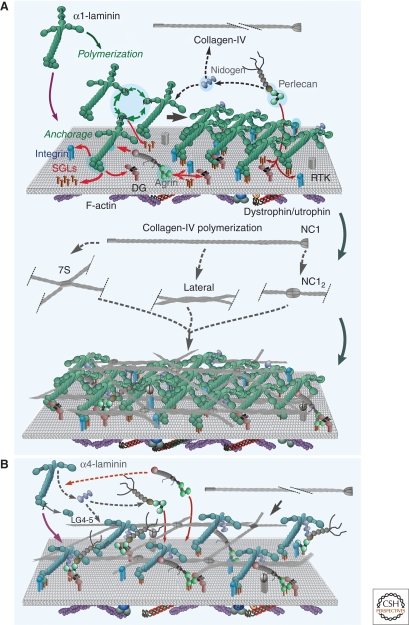
Figure 3.
Basement membrane assembly. (A) Steps in the assembly of a basement membrane initiated by a polymerizing laminin. The laminin LG domains bind to a competent cell surface through sulfated glycolipids (SGL), available integrins and α-dystroglycan, promoting laminin polymerization through its LN domains. The α-LN domain also binds to sulfatides and integrins, forcing the laminin onto its side and enabling activation of a new subset of integrins. Nidogens bind to the coiled-coil domain of laminin and to type IV collagen, forming a stabilizing bridge (the collagen also binds to the developing basement membrane through other poorly characterized interactions). Type IV collagen polymerizes to form a second covalently stabilized network. Agrin and perlecan bind to the laminin coiled-coil and to nidogen respectively and also bind to dystroglycan (DG), integrins, and sulfated glycolipids, establishing collateral linkages to additional receptors. Heparin-binding growth factors (GF) bind to the heparan sulfate chains and to their receptor tyrosine kinases (RTK), activating signaling pathways in concert with integrin activation. (B) Steps in the assembly of a basement membrane initiated by a nonpolymerizing laminin. In the case of α4-laminin with weak LG interactions and no α-LN domain, anchorage may depend heavily on collateral linkage through agrin and perlecan. The laminin establishes links to type IV collagen through nidogen; however, in the absence of polymerization, the resulting scaffold has a lower laminin density.