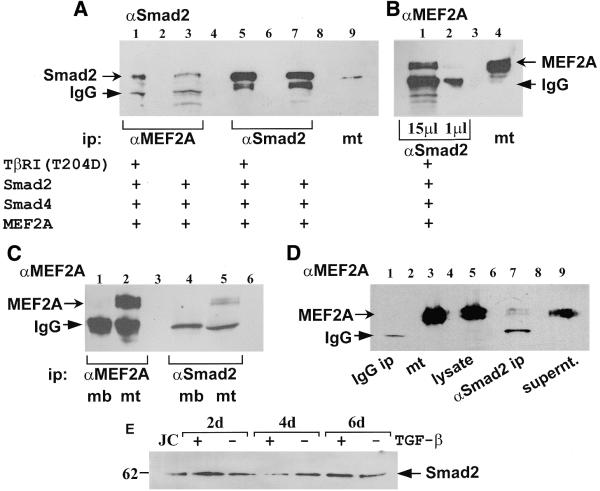
Figure 3.
A physical interaction between endogenous Smad2 and MEF2A in C2C12 mt was detected through co-immunoprecipitation. (A and B) COS cells were transiently transfected with pCMV5B–TβRI(T204D), pCMV5B–Smad2, pCMV5B–Smad4 and pMT2–MEF2A as indicated. Cell lysates were immunoprecipitated with antibodies raised against either MEF2A (α-MEF2A), or Smad2 (α-Smad2). (A) An immunoblot probed by an α-Smad2 antibody contains: 15 µl of anti-MEF2A immunoprecipitate (α-MEF2A ip) in lanes 1 and 3; 15 µl of anti-Smad2 immunoprecipitate (α-Smad2 ip) in lanes 5 and 7; and 30 µg of C2C12 cell extract in lane 9. The arrow indicates Smad2 protein. (B) An immunoblot probed by an α-MEF2A antibody contains: 15 µl of α-Smad2 ip in lane 1; 1 µl of α-Smad2 ip in lane 2; 20 µg of C2C12 cell extract in lane 4. Lane 3 was empty (C) An immunoblot probed by an α-MEF2A antibody contains: a molecular weight marker in lane 1; 1 µl of α-MEF2A ip from myoblast lysate (mb) in lane 2; 1 µl of α-MEF2A ip from myotube lysate (mt) in lane 3; 20 µl of α-Smad2 ip from mb in lane 5; and 20 µl of α-Smad2 ip from mt in lane 6. (D) A negative control for the co-immunoprecipitation assays was performed. An immunoblot probed with an α-MEF2A antibody contains: 20 µl of α-mouse IgG ip from mt lysate in lane 1; 50 µg of mt cellular extract (mt) in lane 3; 1 µl of mt lysate in lane 5 (used for α-Smad2 ip in lane 7); 20 µl of α-Smad2 ip from mt in lane 7; 15 µl of pre-cleared supernatant from α-Smad2 ip (lane 7) in lane 9; and a molecular weight marker in lane 10. Lanes 2, 4, 6 and 8 were empty. (B–D) The arrow indicates MEF2A protein. (A–D) The arrowheads indicate IgG recognised by the goat anti-rabbit secondary antibody. The bands below the IgG are non-specific. (E) C2C12 mb were plated at 25% confluence and 24 h later the medium was replaced with differentiation medium with (+) or without (–) 2 ng/ml TGF-β. After 2, 4 and 6 days (d), protein expression was analysed by loading 20 µg of each extract on a SDS–polyacrylamide gel and western blotting using an anti-Smad2 antibody. Jurkats cell extract (JC) was used as a control.