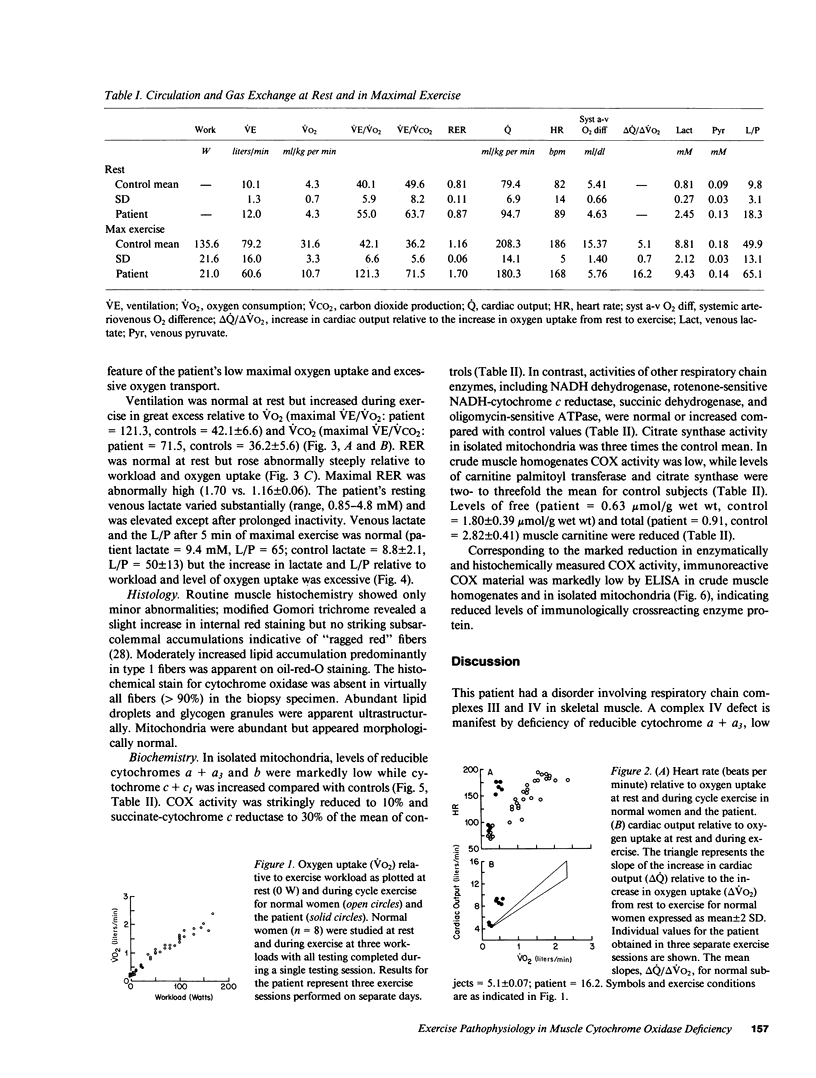
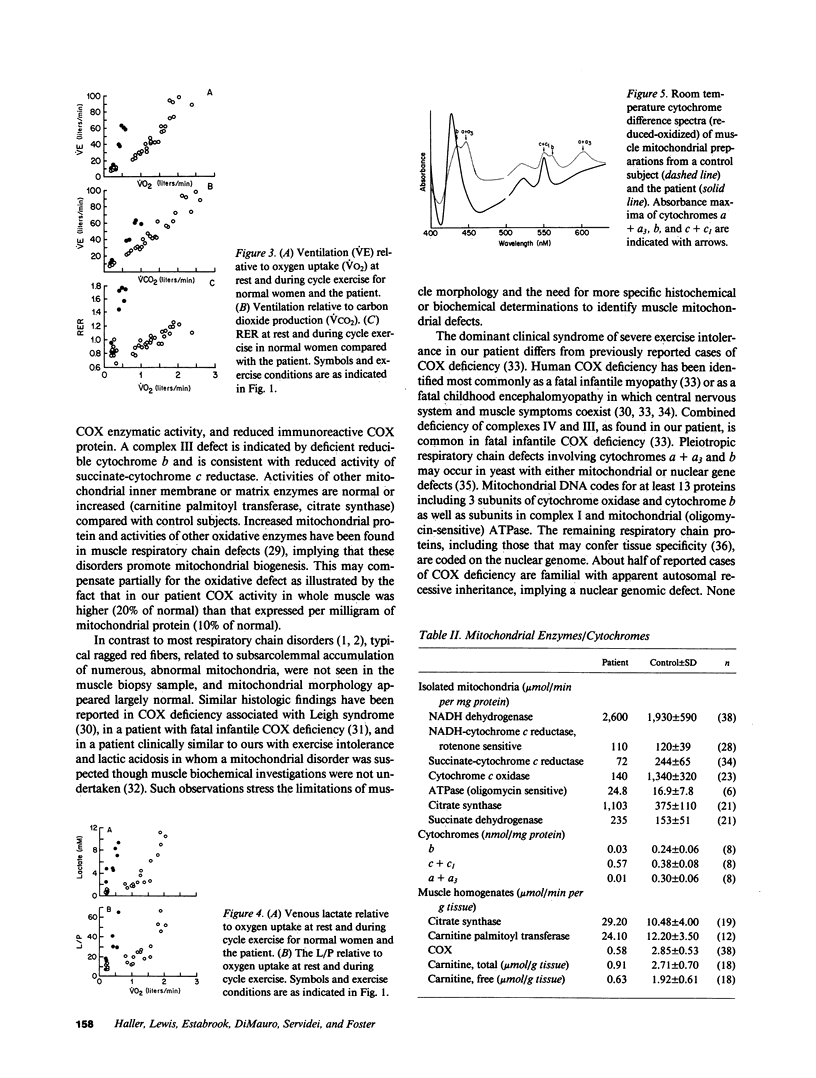
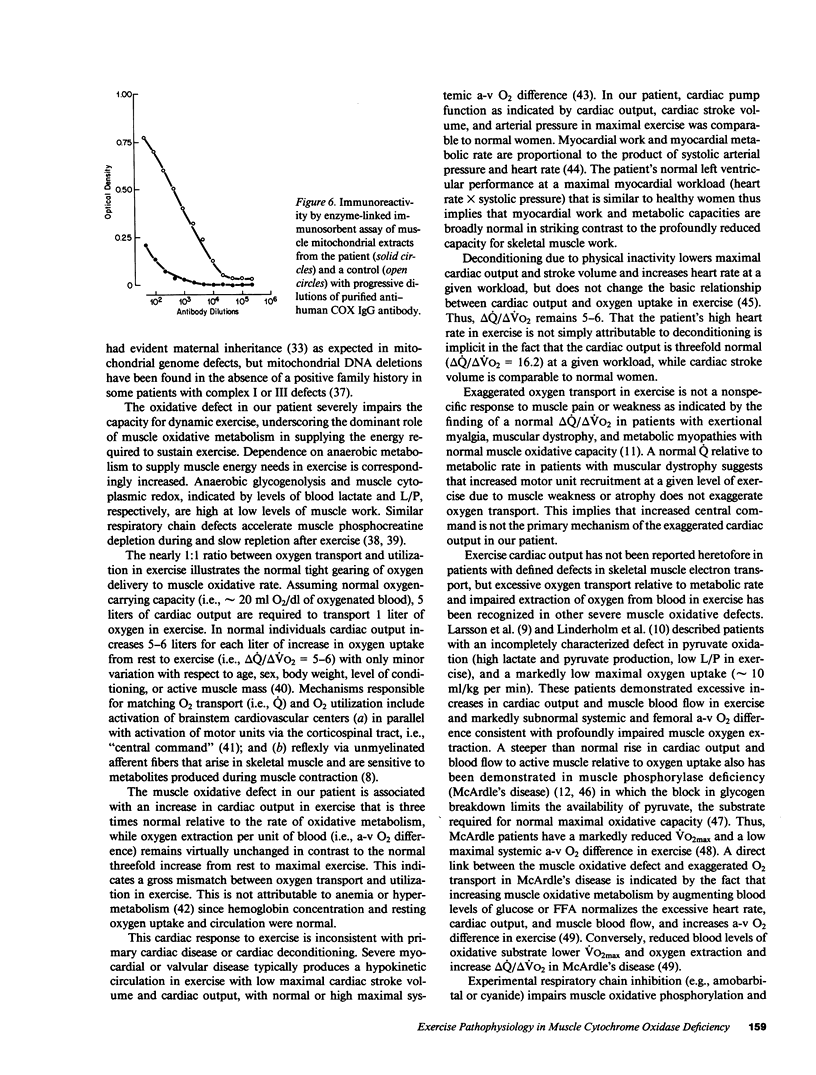
Abstract
A 27-yr-old woman with lifelong severe exercise intolerance manifested by muscle fatigue, lactic acidosis, and prominent symptoms of dyspnea and tachycardia induced by trivial exercise was found to have a skeletal muscle respiratory chain defect characterized by low levels of reducible cytochromes a + a3 and b in muscle mitochondria and marked deficiency of cytochrome c oxidase (complex IV) as assessed biochemically and immunologically. Investigation of the pathophysiology of the exercise response in the patient revealed low maximal oxygen uptake (1/3 that of normal sedentary women) in cycle exercise and impaired muscle oxygen extraction as indicated by profoundly low maximal systemic arteriovenous oxygen difference (5.8 ml/dl; controls = 15.4 +/- 1.4, mean +/- SD). The increases in cardiac output and ventilation during exercise, normally closely coupled to muscle metabolic rate, were markedly exaggerated (more than two- to threefold normal) relative to oxygen uptake and carbon dioxide production accounting for prominent tachycardia and dyspnea at low workloads. Symptoms in our patient are similar to those reported in other human skeletal muscle respiratory chain defects involving complexes I and III, and the exaggerated circulatory response resembles that seen during experimental inhibition of the mitochondrial respiratory chain. These results suggest that impaired oxidative phosphorylation in working muscle disrupts the normal regulation of cardiac output and ventilation relative to muscle metabolic rate in exercise.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arts W. F., Scholte H. R., Loonen M. C., Przyrembel H., Fernandes J., Trijbels J. M., Luyt-Houwen I. E. Cytochrome c oxidase deficiency in subacute necrotizing encephalomyelopathy. J Neurol Sci. 1987 Jan;77(1):103–115. doi: 10.1016/0022-510x(87)90211-5. [DOI] [PubMed] [Google Scholar]
- Bookelman H., Trijbels J. M., Sengers R. C., Janssen A. J. Measurement of cytochromes in human skeletal muscle mitochondria, isolated from fresh and frozen stored muscle specimens. Biochem Med. 1978 Jun;19(3):366–373. doi: 10.1016/0006-2944(78)90037-6. [DOI] [PubMed] [Google Scholar]
- Bresolin N., Zeviani M., Bonilla E., Miller R. H., Leech R. W., Shanske S., Nakagawa M., DiMauro S. Fatal infantile cytochrome c oxidase deficiency: decrease of immunologically detectable enzyme in muscle. Neurology. 1985 Jun;35(6):802–812. doi: 10.1212/wnl.35.6.802. [DOI] [PubMed] [Google Scholar]
- Chance B. Applications of 31P NMR to clinical biochemistry. Ann N Y Acad Sci. 1984;428:318–332. doi: 10.1111/j.1749-6632.1984.tb12307.x. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Bonilla E., Zeviani M., Nakagawa M., DeVivo D. C. Mitochondrial myopathies. Ann Neurol. 1985 Jun;17(6):521–538. doi: 10.1002/ana.410170602. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Servidei S., Zeviani M., DiRocco M., DeVivo D. C., DiDonato S., Uziel G., Berry K., Hoganson G., Johnsen S. D. Cytochrome c oxidase deficiency in Leigh syndrome. Ann Neurol. 1987 Oct;22(4):498–506. doi: 10.1002/ana.410220409. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Zeviani M., Servidei S., Bonilla E., Miranda A. F., Prelle A., Schon E. A. Cytochrome oxidase deficiency: clinical and biochemical heterogeneity. Ann N Y Acad Sci. 1986;488:19–32. doi: 10.1111/j.1749-6632.1986.tb46545.x. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L., Millhorn D. E., Waldrop T. G. Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science. 1981 Feb 20;211(4484):844–846. doi: 10.1126/science.7466362. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D. Metabolism of substrates: energy substrate metabolism during exercise and as modified by training. Fed Proc. 1985 Feb;44(2):353–357. [PubMed] [Google Scholar]
- Haller R. G., Lewis S. F. Abnormal ventilation during exercise in McArdle's syndrome: modulation by substrate availability. Neurology. 1986 May;36(5):716–719. doi: 10.1212/wnl.36.5.716. [DOI] [PubMed] [Google Scholar]
- Haller R. G., Lewis S. F., Cook J. D., Blomqvist C. G. Hyperkinetic circulation during exercise in neuromuscular disease. Neurology. 1983 Oct;33(10):1283–1287. doi: 10.1212/wnl.33.10.1283. [DOI] [PubMed] [Google Scholar]
- Hayes D. J., Lecky B. R., Landon D. N., Morgan-Hughes J. A., Clark J. B. A new mitochondrial myopathy. Biochemical studies revealing a deficiency in the cytochrome b-c1 complex (complex III) of the respiratory chain. Brain. 1984 Dec;107(Pt 4):1165–1177. doi: 10.1093/brain/107.4.1165. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Morgan-Hughes J. A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988 Feb 25;331(6158):717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- Hoppel C. L., Kerr D. S., Dahms B., Roessmann U. Deficiency of the reduced nicotinamide adenine dinucleotide dehydrogenase component of complex I of mitochondrial electron transport. Fatal infantile lactic acidosis and hypermetabolism with skeletal-cardiac myopathy and encephalopathy. J Clin Invest. 1987 Jul;80(1):71–77. doi: 10.1172/JCI113066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennaway N. G., Buist N. R., Darley-Usmar V. M., Papadimitriou A., Dimauro S., Kelley R. I., Capaldi R. A., Blank N. K., D'Agostino A. Lactic acidosis and mitochondrial myopathy associated with deficiency of several components of complex III of the respiratory chain. Pediatr Res. 1984 Oct;18(10):991–999. doi: 10.1203/00006450-198410000-00017. [DOI] [PubMed] [Google Scholar]
- Kuhn-Nentwig L., Kadenbach B. Isolation and properties of cytochrome c oxidase from rat liver and quantification of immunological differences between isozymes from various rat tissues with subunit-specific antisera. Eur J Biochem. 1985 May 15;149(1):147–158. doi: 10.1111/j.1432-1033.1985.tb08905.x. [DOI] [PubMed] [Google Scholar]
- LARSSON L. E., LINDERHOLM H., MUELLER R., RINGQVIST T., SOERNAES R. HEREDITARY METABOLIC MYOPATHY WITH PAROXYSMAL MYOGLOBINURIA DUE TO ABNORMAL GLYCOLYSIS. J Neurol Neurosurg Psychiatry. 1964 Oct;27:361–380. doi: 10.1136/jnnp.27.5.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Land J. M., Morgan-Hughes J. A., Clark J. B. Mitochondrial myopathy. Biochemical studies revealing a deficiency of NADH--cytochrome b reductase activity. J Neurol Sci. 1981 Apr;50(1):1–13. doi: 10.1016/0022-510x(81)90038-1. [DOI] [PubMed] [Google Scholar]
- Lewis S. F., Haller R. G., Cook J. D., Blomqvist C. G. Metabolic control of cardiac output response to exercise in McArdle's disease. J Appl Physiol Respir Environ Exerc Physiol. 1984 Dec;57(6):1749–1753. doi: 10.1152/jappl.1984.57.6.1749. [DOI] [PubMed] [Google Scholar]
- Lewis S. F., Haller R. G., Cook J. D., Nunnally R. L. Muscle fatigue in McArdle's disease studied by 31P-NMR: effect of glucose infusion. J Appl Physiol (1985) 1985 Dec;59(6):1991–1994. doi: 10.1152/jappl.1985.59.6.1991. [DOI] [PubMed] [Google Scholar]
- Lewis S. F., Haller R. G. The pathophysiology of McArdle's disease: clues to regulation in exercise and fatigue. J Appl Physiol (1985) 1986 Aug;61(2):391–401. doi: 10.1152/jappl.1986.61.2.391. [DOI] [PubMed] [Google Scholar]
- Lewis S. F., Taylor W. F., Graham R. M., Pettinger W. A., Schutte J. E., Blomqvist C. G. Cardiovascular responses to exercise as functions of absolute and relative work load. J Appl Physiol Respir Environ Exerc Physiol. 1983 May;54(5):1314–1323. doi: 10.1152/jappl.1983.54.5.1314. [DOI] [PubMed] [Google Scholar]
- Liang C. S., Hood W. B., Jr Afferent neural pathway in the regulation of cardiopulmonary responses to tissue hypermetabolism. Circ Res. 1976 Mar;38(3):209–214. doi: 10.1161/01.res.38.3.209. [DOI] [PubMed] [Google Scholar]
- Liang C., Huckabee W. E. Mechanisms regulating the cardiac output response to cyanide infusion, a model of hypoxia. J Clin Invest. 1973 Dec;52(12):3115–3128. doi: 10.1172/JCI107511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderholm H., Müller R., Ringqvist T., Sörnäs R. Hereditary abnormal muscle metabolism with hyperkinetic circulation during exercise. Acta Med Scand. 1969 Mar;185(3):153–166. doi: 10.1111/j.0954-6820.1969.tb07314.x. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Lee C. P. Biochemical studies of skeletal muscle mitochondria. I. Microanalysis of cytochrome content, oxidative and phosphorylative activities of mammalian skeletal muscle mitochondria. Arch Biochem Biophys. 1968 Jul;126(1):75–82. doi: 10.1016/0003-9861(68)90561-4. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Leatherman G. F., Foster D. W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978 Jun 25;253(12):4128–4136. [PubMed] [Google Scholar]
- Mitchell J. H., Kaufman M. P., Iwamoto G. A. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Morgan-Hughes J. A., Darveniza P., Kahn S. N., Landon D. N., Sherratt R. M., Land J. M., Clark J. B. A mitochondrial myopathy characterized by a deficiency in reducible cytochrome b. Brain. 1977 Dec;100(4):617–640. doi: 10.1093/brain/100.4.617. [DOI] [PubMed] [Google Scholar]
- Mukherjee A., Krishnamurty V. S. Effect of beta-diethylaminoethyl-diphenylpropylacetate HCl (SKF 525-A) on canine heart mitochondrial function. Biochem Pharmacol. 1980 Feb;29(3):283–288. doi: 10.1016/0006-2952(80)90501-8. [DOI] [PubMed] [Google Scholar]
- Nuutinen E. M., Nishiki K., Erecińska M., Wilson D. F. Role of mitochondrial oxidative phosphorylation in regulation of coronary blood flow. Am J Physiol. 1982 Aug;243(2):H159–H169. doi: 10.1152/ajpheart.1982.243.2.H159. [DOI] [PubMed] [Google Scholar]
- Olson W., Engel W. K., Walsh G. O., Einaugler R. Oculocraniosomatic neuromuscular disease with "ragged-red" fibers. Arch Neurol. 1972 Mar;26(3):193–211. doi: 10.1001/archneur.1972.00490090019001. [DOI] [PubMed] [Google Scholar]
- Petty R. K., Harding A. E., Morgan-Hughes J. A. The clinical features of mitochondrial myopathy. Brain. 1986 Oct;109(Pt 5):915–938. doi: 10.1093/brain/109.5.915. [DOI] [PubMed] [Google Scholar]
- Radda G. K., Bore P. J., Gadian D. G., Ross B. D., Styles P., Taylor D. J., Morgan-Hughes J. 31P NMR examination of two patients with NADH-CoQ reductase deficiency. Nature. 1982 Feb 18;295(5850):608–609. doi: 10.1038/295608a0. [DOI] [PubMed] [Google Scholar]
- Rimoldi M., Bottacchi E., Rossi L., Cornelio F., Uziel G., Di Donato S. Cytochrome-C-oxidase deficiency in muscles of a floppy infant without mitochondrial myopathy. J Neurol. 1982;227(4):201–207. doi: 10.1007/BF00313387. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman K. E., Alfrey A., Kirsch W. M., Zweig P., Felig P., Messner F. Chronic lactic acidosis in an adult. A new syndrome associated with an altered redox state of certain NAD-NADH coupled reactions. Am J Med. 1970 Jan;48(1):104–112. doi: 10.1016/0002-9343(70)90104-x. [DOI] [PubMed] [Google Scholar]
- Triebwasser J. H., Johnson R. L., Burpo R. P., Campbell J. C., Reardon W. C., Blomqvist C. G. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Environ Med. 1977 Mar;48(3):203–209. [PubMed] [Google Scholar]
- Wilson J. R., Martin J. L., Schwartz D., Ferraro N. Exercise intolerance in patients with chronic heart failure: role of impaired nutritive flow to skeletal muscle. Circulation. 1984 Jun;69(6):1079–1087. doi: 10.1161/01.cir.69.6.1079. [DOI] [PubMed] [Google Scholar]



