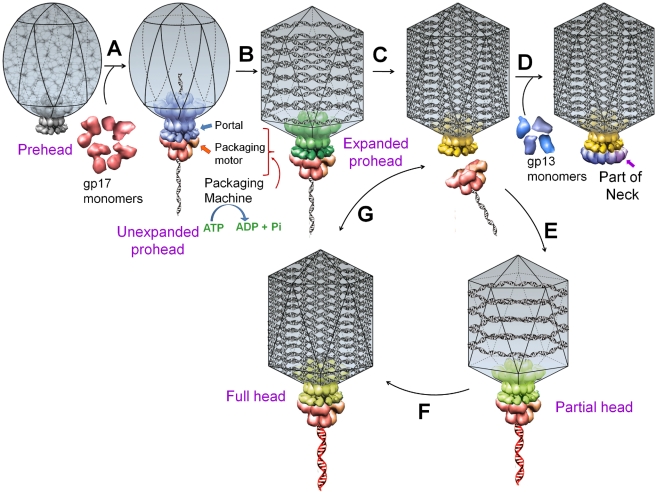
Figure 1. A schematic of DNA packaging by sequential assembly and promiscuous assembly.
The major capsid protein assembles around a scaffolding core into a prehead. The core is removed by proteolysis to produce an empty unexpanded prohead (A). The unexpanded prohead normally has a round shape, but in phage T4 it has angular geometry [45]. The packaging motor–DNA complex docks on portal and initiates packaging. The prohead expands after about 10%–25% of the DNA is packaged (B). After headful packaging, the motor cuts the concatemeric DNA and dissociates from the DNA-full head (C). The neck proteins (gp13, gp14, and gp15) assemble on portal to seal off the DNA-full head and provide a platform for tail assembly (D). The various colors of portal represent different conformational states. In promiscuous assembly, the packaging motor assembles on a partial head produced by ejection of packaged DNA (E) or a full head (G), and refills the head with new fragments of DNA ([F] and [G]; new DNA fragments shown in red).