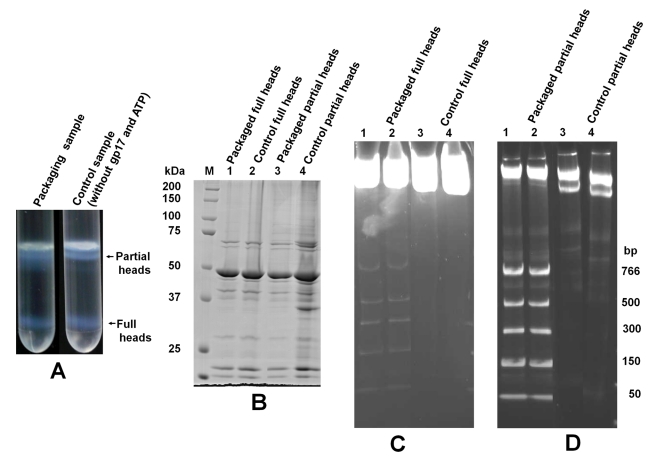
Figure 4. Full heads can package DNA.
(A) The phage heads were isolated from 10am13am infected E. coli P301 cells (500 ml culture) by lysis in the presence of DNAse I followed by differential centrifugation (see Materials and Methods for details). The phage head pellet containing a mixture of partial heads and full heads was resuspended in 200 µl of 50 mM Tris-HCl (pH 7.5) and 5 mM MgCl2. The sample was split into two halves, and larger scale packaging assays were conducted immediately. The 500-µl packaging reactions contained 100 µl of phage heads, 4.75 µM GFP-gp17, 43 µg of ladder DNA (50–766 bp; NEB), 5% PEG buffer, and 1 mM ATP [30]. gp17 and ATP were omitted in the control reaction. After 30 min of incubation at room temperature, 40 µl (1,000 units) of Benzonase nuclease (EMD Biosciences) was added to digest unpackaged DNA, and the samples were separated by CsCl density gradient centrifugation. (B) The partial and full head samples from the gradient were electrophoresed on 10% SDS polyacrylamide gel to analyze for proteins and to estimate the concentration of particles used in the packaging reactions. Since the concentration of full heads is very low compared to that of partial heads (roughly 1/10th that of partial heads), the full heads were concentrated by high-speed centrifugation such that the number of particles per lane are approximately the same for both full heads and partial heads. (C and D) The full (C) and partial (D) head bands from the gradient were treated with proteinase K (18.5 µg; Fermentas) and electrophoresed on 4%–20% polyacrylamide gel in Tris-borate buffer (pH 8) to analyze for packaged DNA.