Figure 4.
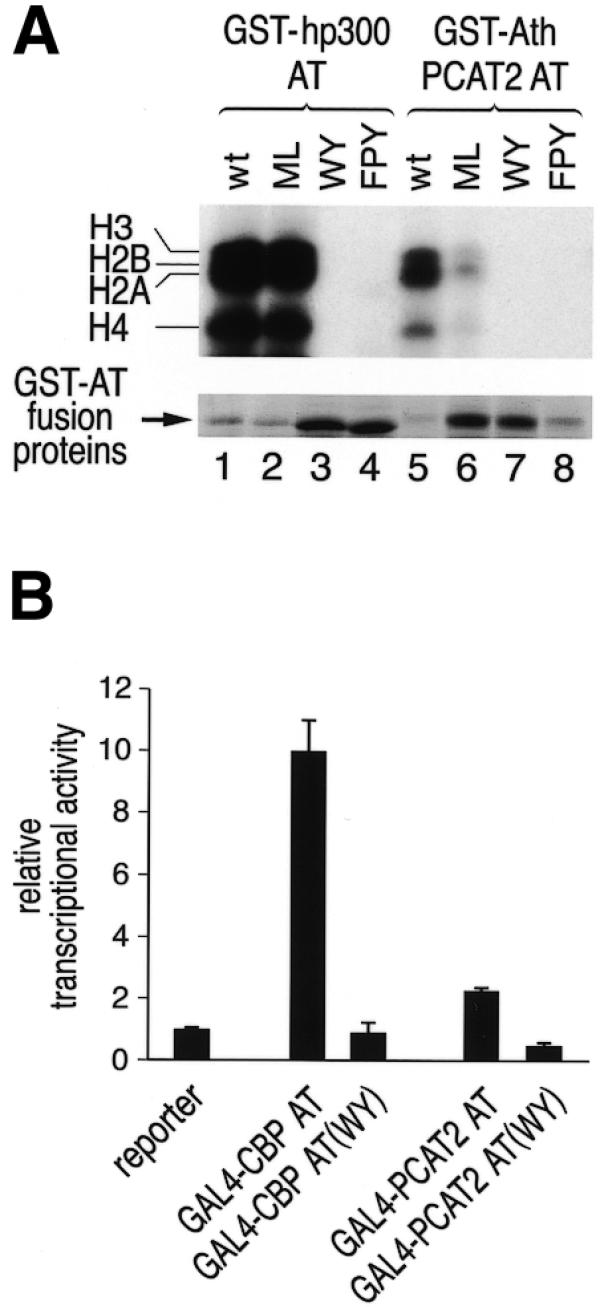
Conserved residues which are essential for the AT activity of human p300 and CBP are also essential for the enzymatic activity of Arabidopsis PCAT2 and for stimulation of transcription by the respective AT domains. (A) Specific point mutations were introduced into the GST–AT domain fusion proteins of both species (see Fig. 1B and Materials and Methods for exact location of mutations) and HAT assays were performed. The top panel shows an autoradiograph of the SDS–PAGE gel demonstrating that mutation of FPY or WY residues abolished AT activity of both human p300 and plant PCAT2. By contrast, mutation of the residues ML did not abolish AT activity of hp300 and reduced activity of the plant protein by a factor of about 3. The bottom panel shows a Coomassie blue stained section of the same protein gel revealing expression levels of the hp300 and PCAT2 GST–AT fusion proteins. (B) U2OS cells were transiently transfected with a GAL4-dependent reporter gene and GAL4–AT expression plasmids. GAL4–CBP AT strongly activated transcription of the reporter plasmid in a manner dependent on AT activity since GAL4–CBP (WY) was inactive. In comparison, GAL4–PCAT2 AT also activated transcription in an AT-dependent manner, but less efficently than the CBP AT domain.
