Figure 5.
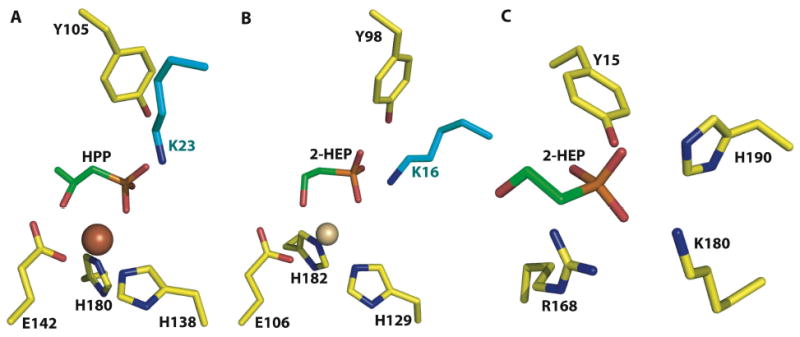
A close-up view of the actives sites of (A) HppE in complex with 2-HPP, (B) HEPD in complex with 2-HEP, and (C) DhpI in complex with 2-HEP. Despite the lack of any notable sequence similarities, both HEPD and HppE use a near identical constellation of active site residues to carry out their respective reactions. Notably, both polypeptides contain composite active sites with a catalytically essential lysine residue (colored in cyan) from a different subunit interacting with the active site. A comparison of HppE, HEPD, and DhpI co-crystal structures illustrates the chemical features that are used by functionally and structurally distinct enzymes to harbor phosphonate substrates.
