Figure 6.
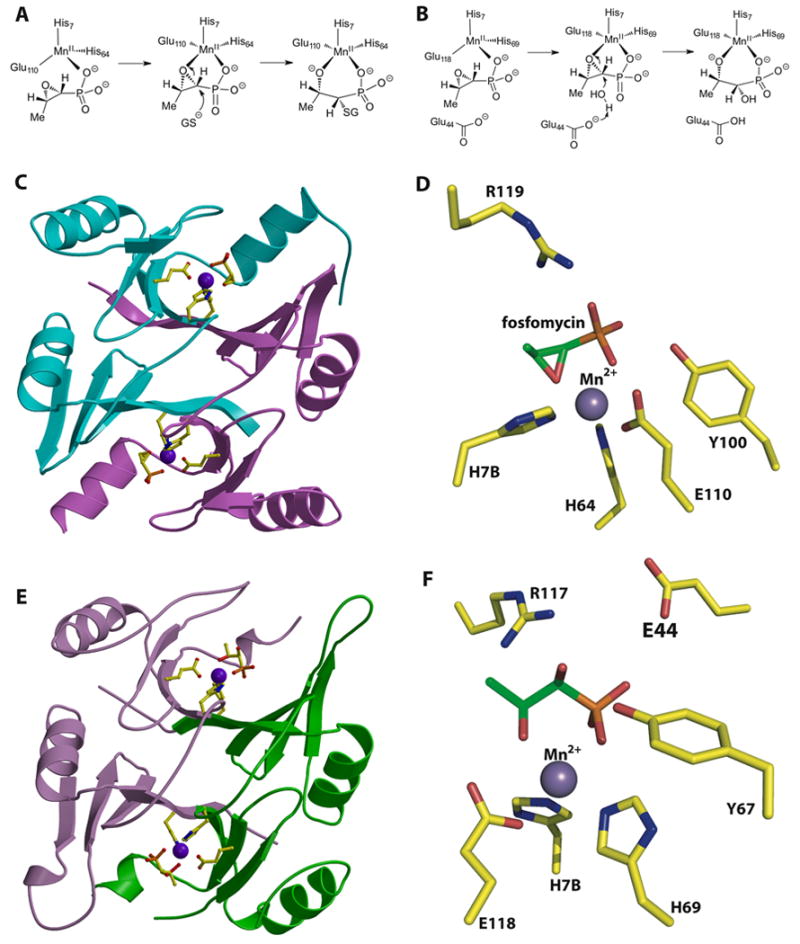
(A) Reaction catalyzed by the fosfomycin thiol transferase FosA. (B) Reaction catalyzed by the homologous FosX metalloenzyme that hydrates the antibiotic. (C) Structure of the fosfomycin inactivating enzyme FosA. (D) Close-up view of the active site of FosA. (E) Structure of the fosfomycin hydrolase FosX. (F) Close-up view of the active site of FosX revealing a glutamate residue (Glu44) that is proximal to the substrate and that is essential for catalysis. This residue is absent in FosA.
