Abstract
The removal of neutrophils and their histotoxic contents from the inflamed site is a prerequisite for resolution of tissue injury, and a point at which factors critical to the pathogenesis of chronic inflammation may act. Engulfment of intact, senescent neutrophils by macrophages represents an important neutrophil disposal process. In this study the mechanism by which human monocyte-derived macrophages (M phi) recognized and ingested human neutrophils that had been aged in culture was studied using an in vitro phagocytic assay. Inhibition of M phi receptors for Ig Fc and the opsonic complement fragments C3b and iC3b with MAbs to M phi FcR, CR1, CR3, and CR4 had no effect on recognition, and the pattern of inhibition observed when polyanions were included in the medium at 1 mg/ml was different from that reported for the M phi receptor for protein advanced glycosylation end products (AGE), indicating a recognition mechanism different from those proposed for M phi phagocytosis of senescent erythrocytes. Furthermore, although aging neutrophils undergo programmed cell death (or apoptosis), which is directly related to recognition by M phi, the pattern of inhibition observed with monosaccharides was different from that reported to inhibit the binding of apoptotic mouse thymocytes to isologous M phi. By contrast, evidence was obtained for a novel recognition mechanism inhibitable by cationic sugars and amino acids in a charge-dependent fashion, and directly modulated by pH but not affected by inhibitors of the mannose-6-phosphate, sheep erythrocyte, mannosyl-fucosyl, asialoglycoprotein, and scavenger receptors of the macrophage. These observations suggest that hydrogen ions and charged molecules may modulate M phi uptake of senescent neutrophils at inflamed sites, and that recognition itself may involve charged structures on the cells.
Full text
PDF

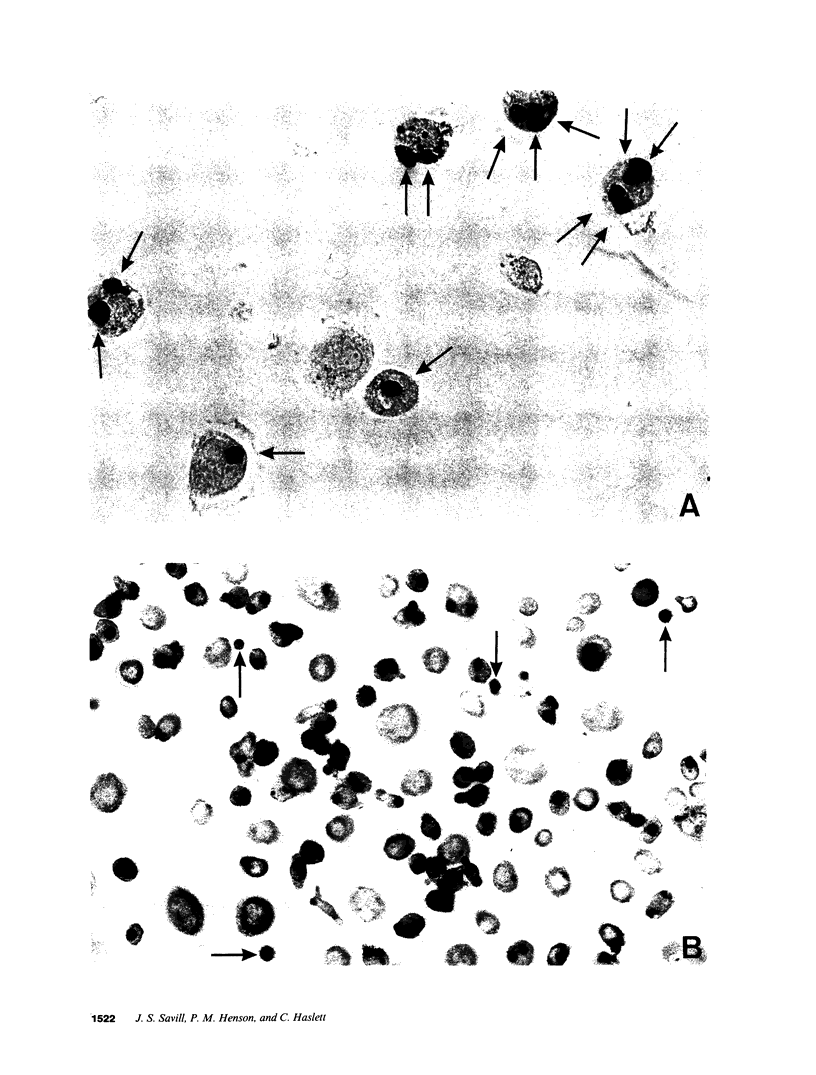

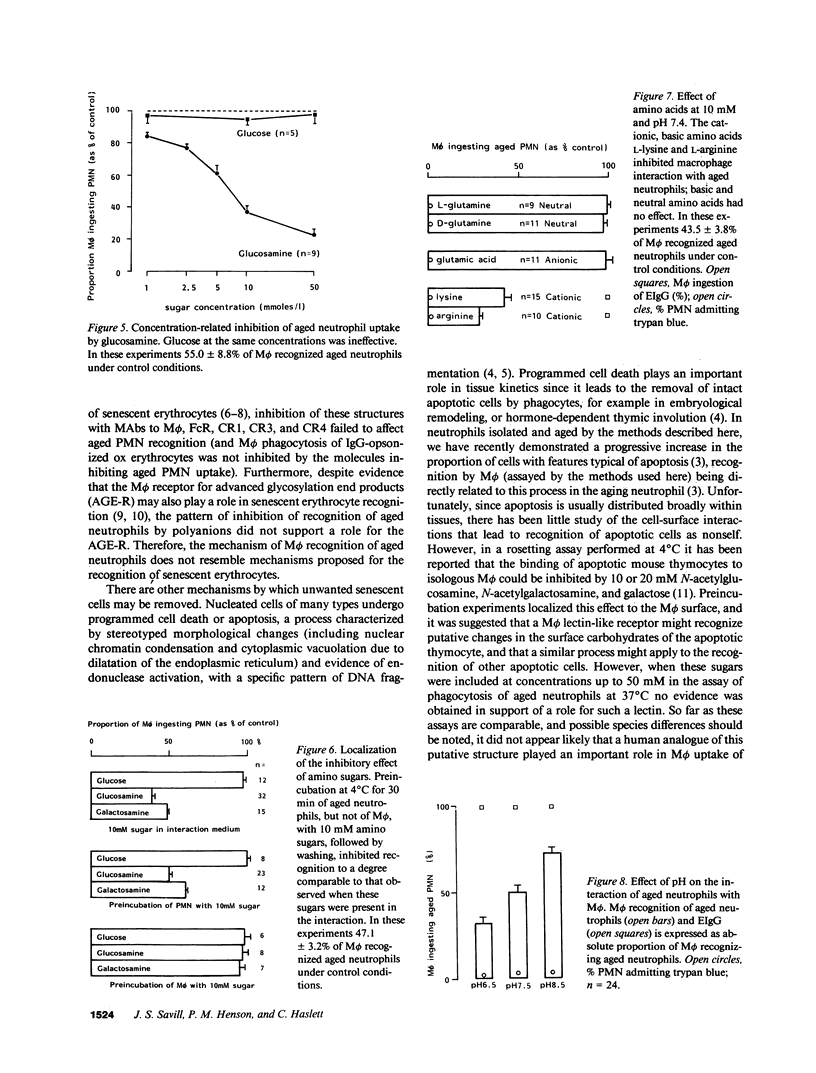



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREWER D. B. ELECTRON-MICROSCOPE OBSERVATIONS ON THE PHAGOCYTOSIS OF NEUTROPHIL POLYMORPHONUCLEAR LEUCOCYTES BY MACROPHAGES. J Pathol Bacteriol. 1964 Jul;88:307–309. doi: 10.1002/path.1700880139. [DOI] [PubMed] [Google Scholar]
- Blackwell J. M., Ezekowitz R. A., Roberts M. B., Channon J. Y., Sim R. B., Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985 Jul 1;162(1):324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Basu S. K., Falck J. R., Ho Y. K., Goldstein J. L. The scavenger cell pathway for lipoprotein degradation: specificity of the binding site that mediates the uptake of negatively-charged LDL by macrophages. J Supramol Struct. 1980;13(1):67–81. doi: 10.1002/jss.400130107. [DOI] [PubMed] [Google Scholar]
- Chapes S. K., Haskill S. Evidence for granulocyte-mediated macrophage activation after C. parvum immunization. Cell Immunol. 1983 Feb 1;75(2):367–377. doi: 10.1016/0008-8749(83)90334-9. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G. Immunologic tissue injury mediated by neutrophilic leukocytes. Adv Immunol. 1968;9:97–162. doi: 10.1016/s0065-2776(08)60442-3. [DOI] [PubMed] [Google Scholar]
- Crocker P. R., Gordon S. Properties and distribution of a lectin-like hemagglutinin differentially expressed by murine stromal tissue macrophages. J Exp Med. 1986 Dec 1;164(6):1862–1875. doi: 10.1084/jem.164.6.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Morris L., Gordon S. Novel cell surface adhesion receptors involved in interactions between stromal macrophages and haematopoietic cells. J Cell Sci Suppl. 1988;9:185–206. doi: 10.1242/jcs.1988.supplement_9.10. [DOI] [PubMed] [Google Scholar]
- Duvall E., Wyllie A. H., Morris R. G. Macrophage recognition of cells undergoing programmed cell death (apoptosis). Immunology. 1985 Oct;56(2):351–358. [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Stahl P. D. The structure and function of vertebrate mannose lectin-like proteins. J Cell Sci Suppl. 1988;9:121–133. doi: 10.1242/jcs.1988.supplement_9.6. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Rasmussen R. R., Olch C. L., Fogelman A. M. Two distinct receptors account for recognition of maleyl-albumin in human monocytes during differentiation in vitro. J Clin Invest. 1986 Mar;77(3):681–689. doi: 10.1172/JCI112362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr, Henson P. M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985 Apr;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Johnston R. B., Jr Tissue injury in inflammation. Oxidants, proteinases, and cationic proteins. J Clin Invest. 1987 Mar;79(3):669–674. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Zanolari B., Schwartzman N. A., Hong S. R. Intracellular control of human neutrophil secretion. I. C5a-induced stimulus-specific desensitization and the effects of cytochalasin B. J Immunol. 1978 Sep;121(3):851–855. [PubMed] [Google Scholar]
- Hoflack B., Kornfeld S. Lysosomal enzyme binding to mouse P388D1 macrophage membranes lacking the 215-kDa mannose 6-phosphate receptor: evidence for the existence of a second mannose 6-phosphate receptor. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4428–4432. doi: 10.1073/pnas.82.13.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T. K., Banda M. J., Silver I. A. Cell interactions in post-traumatic fibrosis. Ciba Found Symp. 1985;114:127–149. doi: 10.1002/9780470720950.ch9. [DOI] [PubMed] [Google Scholar]
- Kay M. M. Mechanism of removal of senescent cells by human macrophages in situ. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3521–3525. doi: 10.1073/pnas.72.9.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Role of physiologic autoantibody in the removal of senescent human red cells. J Supramol Struct. 1978;9(4):555–567. doi: 10.1002/jss.400090409. [DOI] [PubMed] [Google Scholar]
- Kolb-Bachofen V., Schlepper-Schäfer J., Vogell W., Kolb H. Electron microscopic evidence for an asialoglycoprotein receptor on Kupffer cells: localization of lectin-mediated endocytosis. Cell. 1982 Jul;29(3):859–866. doi: 10.1016/0092-8674(82)90447-0. [DOI] [PubMed] [Google Scholar]
- Myones B. L., Dalzell J. G., Hogg N., Ross G. D. Neutrophil and monocyte cell surface p150,95 has iC3b-receptor (CR4) activity resembling CR3. J Clin Invest. 1988 Aug;82(2):640–651. doi: 10.1172/JCI113643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Henson J. E., Henson P. M. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982 Aug 1;156(2):430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekin T. J., Jr, Malinin T. I., Zvaifler N. J. Unusual synovial fluid findings in Reiter's syndrome. Ann Intern Med. 1967 Apr;66(4):677–684. doi: 10.7326/0003-4819-66-4-677. [DOI] [PubMed] [Google Scholar]
- Riches D. W., Stanworth D. R. Evidence for a mechanism for the initiation of acid hydrolase secretion by macrophages that is functionally independent of alternative pathway complement activation. Biochem J. 1982 Mar 15;202(3):639–645. doi: 10.1042/bj2020639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos P. H., Hartman H. J., Schlepper-Schäfer J., Kolb H., Kolb-Bachofen V. Galactose-specific receptors on liver cells. II. Characterization of the purified receptor from macrophages reveals no structural relationship to the hepatocyte receptor. Biochim Biophys Acta. 1985 Oct 30;847(1):115–121. doi: 10.1016/0167-4889(85)90161-2. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- Sanui H., Yoshida S., Nomoto K., Ohhara R., Adachi Y. Peritoneal macrophages which phagocytose autologous polymorphonuclear leucocytes in guinea-pigs. I: induction by irritants and microorgansisms and inhibition by colchicine. Br J Exp Pathol. 1982 Jun;63(3):278–284. [PMC free article] [PubMed] [Google Scholar]
- Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989 Mar;83(3):865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlepper-Schäfer J., Kolb-Bachofen V., Kolb H. Analysis of lectin-dependent recognition of desialylated erythrocytes by Kupffer cells. Biochem J. 1980 Mar 15;186(3):827–831. doi: 10.1042/bj1860827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd V. L., Freeze H. H., Miller A. L., Stahl P. D. Identification of mannose 6-phosphate receptors in rabbit alveolar macrophages. J Biol Chem. 1984 Feb 25;259(4):2257–2261. [PubMed] [Google Scholar]
- Spriggs A. I., Boddington M. M., Mowat A. G. Joint fluid cytology in Reiter's disease. Ann Rheum Dis. 1978 Dec;37(6):557–560. doi: 10.1136/ard.37.6.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P., Gordon S. Expression of a mannosyl-fucosyl receptor for endocytosis on cultured primary macrophages and their hybrids. J Cell Biol. 1982 Apr;93(1):49–56. doi: 10.1083/jcb.93.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. Novel macrophage receptor for glucose-modified proteins is distinct from previously described scavenger receptors. J Exp Med. 1986 Oct 1;164(4):1301–1309. doi: 10.1084/jem.164.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Valinsky J., Brownlee M., Cerami C., Nishimoto S., Cerami A. Advanced glycosylation endproducts on erythrocyte cell surface induce receptor-mediated phagocytosis by macrophages. A model for turnover of aging cells. J Exp Med. 1987 Aug 1;166(2):539–549. doi: 10.1084/jem.166.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]