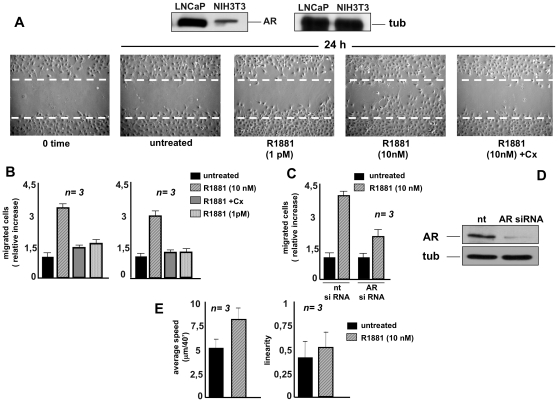
Figure 1. R1881 stimulates migration in NIH3T3 fibroblasts.
Quiescent NIH3T3 cells were used. In A, upper panels: Western blot of lysate proteins from NIH3T3 and prostate cancer-derived LNCaP cells with anti-AR (left) or anti-tubulin (right) antibodies. In A, lower panels: NIH3T3 cells were wounded and then left unstimulated or stimulated for the indicated time with R1881 (at 10 nM or 1 pM). When indicated, Casodex (Cx) was added at a 1000-fold excess. Contrast-phase images are representative of four different experiments, each performed in duplicate. In B, migration assay on collagen-coated (left graph) or uncoated (right graph) Transwell filters was performed in the absence or presence of the indicated compounds. Casodex (Cx) was added at a 1000-fold excess. Migrated cells were stained as described in Methods and data were expressed as relative increase in number of migrated cells. In C and D, growing NIH3T3 cells were transfected with siRNA Alexa Fluor 488 along with either control siRNA (nt) or AR siRNA. After transfection, the cells were made quiescent. In C, the cells were allowed to migrate in collagen-coated Transwell filters in the absence or presence of 10 nM R1881. Migrated cells were stained as described in Methods and data were expressed as relative increase in number of migrated cells. In D, lysate proteins were immunoblotted using the antibodies against the indicated proteins (androgen receptor, AR; tubulin, tub). In E, video time-lapse microscopy was followed in the absence or presence of 10 nM R1881. Data were analyzed as described in Methods and cell motility expressed as average speed (microm/40′; left graph). In both experimental conditions, no significant influence in linearity of cell motility was detected (right graph). In B, C and E, means and SEM are shown; n represents the number of experiments. The statistical significance of results in B, C and E was also evaluated by Student's t test. In B (left and right panels), P values were <0.005 for cells stimulated with 10 nM R1881. No significance was attributed to the difference in relative migration between unstimulated cells and cells stimulated with 10 nM R1881 in the presence of Casodex. Again, no significance was attributed to the difference in relative migration between unstimulated cells and cells stimulated with 1 pM R1881. In C, the difference in relative migration between cells transfected with AR siRNA and those transfected with control siRNA and challenged with 10 nM R1881 was significant (P<0.001). Also significant (P<0.001) was the difference in relative migration between cells transfected with control siRNA and left unstimulated or stimulated with 10 nM R1881. In E, the difference in cell motility between unstimulated cells and cells stimulated with 10 nM R1881 was significant (P<0.005).