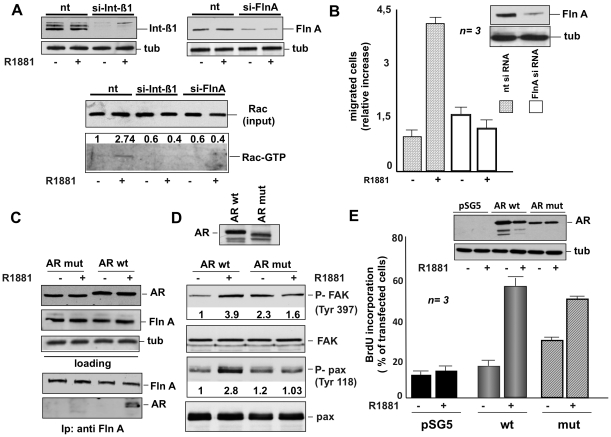
Figure 9. Role of AR, FlnA and integrin beta 1 in androgen-triggered activation of signaling responsible for fibroblast migration.
In A, growing NIH3T3 cells were transfected with control siRNA (nt) or integrin beta 1 siRNA or FlnA siRNA as described in Methods. After transfection, the cells were made quiescent and then left untreated or treated for 5 min with 10 nM R1881. The upper section shows the Western blot of corresponding cell lysates with anti-integrin beta 1 (left) or FlnA (right) antibody. The levels of tubulin were also assessed by Western blot (tub). Cell lysates containing similar amounts of total Rac (input) were used to assay Rac activation in pull-down experiments (Rac-GTP). Data were analyzed using the NIH Image J program and expressed as relative increase. Numbers in the upper portion of corresponding blots represent the increase in Rac-GTP. In B, growing NIH3T3 cells were transfected with siRNA Alexa Fluor 488 along with either control siRNA (nt) or FlnA siRNA. The cells were made quiescent and then allowed to migrate in collagen-coated Transwell filters in the absence or presence of 10 nM R1881. Migrated cells were scored by fluorescent microscope as described in Methods and data expressed as relative increase in number of migrated cells. Results from several independent experiments were collected and analyzed. Means and SEM are shown. n represents the number of experiments. The statistical significance of results was also evaluated by Student's t test. In cells challenged with 10 nM R1881, the difference in relative migration between cells transfected with FlnA siRNA and those transfected with control siRNA was significant (P<0.001). Also significant (P<0.005) was the difference in relative migration between cells transfected with control siRNA and left unstimulated or stimulated with 10 nM R1881. The inset in B shows the Western blot of lysate proteins with anti FlnA (upper) or anti tubulin (lower) antibodies. In C and D, AR-negative Cos-7 cells were transfected with wt human AR (AR wt) or its mutant (AR mut) unable to interact with FlnA. After transfection, the cells were made quiescent and then left untreated or treated for 5 min with 10 nM R1881. In C, lysate proteins containing similar amounts of AR or FlnA or tubulin (upper section) were immunoprecipitated using anti-FlnA antibody. Proteins in immune complexes were analyzed by Western blotting using the antibodies against the indicated proteins (lower section). In D, cell lysates were analyzed by immunoblotting using anti-P-Tyr 397 FAK (P-FAK), FAK (FAK), anti-P-Tyr 118 paxillin (P-Pax), or paxillin (pax) antibodies. Data were analyzed using the NIH Image J program and expressed as relative increase. Numbers in the lower portion of corresponding blots represent the increase in P-FAK or P-pax. Expression of wt AR or its mutant (AR mut) was verified by immunoblotting using the C-19 anti-AR antibody (upper section). In E, AR-negative MDA-MB231 cells on coverslips were transfected with the pSG5 empty plasmid or pSG5- wt AR (AR wt) or pSV1 mutant AR (AR mut). After transfection, the cells were made quiescent and then left unstimulated or stimulated for 18 h with 10 nM R1881. After in vivo pulse with bromodeoxyuridine (BrdU), DNA synthesis was analyzed by IF and calculated by the formula: percentage of BrdU-positive cells = (No. of transfected BrdU-positive cells/No. of transfected cells) X 100. For each plasmid, data were derived from at least 500 transfected cells. Results of three independent experiments were averaged. Means and SEM are shown. n represents the number of experiments. The statistical significance of results was also evaluated by Student' t test. The difference in BrdU incorporation between cells transfected with wt hAR (wt) and those transfected with pSG5 empty vector stimulated with R1881 was significant (P<0.001). Also significant (P<0.001) was the difference in BrdU incorporation between cells transfected with mutant hAR (mut) and those transfected with pSG5 empty vector stimulated with R1881. Lysate proteins were also immunoblotted using the C-19 anti-AR antibody (upper inset in E). The levels of tubulin (tub) were also assessed as a loading control (lower inset in E).