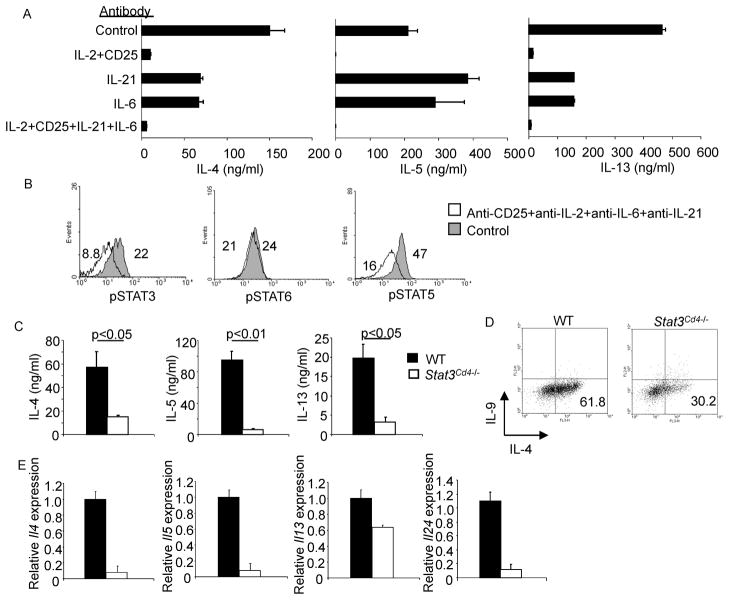
Figure 2. Reduced Th2 Cytokine Production in the Absence of STAT3.
(A) WT naïve CD4+ T cells were activated with anti-CD3 and anti-CD28 and cultured under Th2 conditions, in the presence or absence of antibodies to the indicated cytokines and receptors (20 μg/ml per antibody) for five days before stimulation with anti-CD3. Twenty-four hours after stimulation supernatants were tested for cytokine concentration using ELISA (mean ± s.d.). Results are representative of experiments with four mice.
(B) Cells cultured as in (A) with control antibody or the combination of antibodies indicated were tested for amount of pSTAT by intracellular staining on day 4 of Th2 culture. Numbers represent mean fluorescence intensity. Results are representative of experiments with four mice.
(C) WT and Stat3Cd4−/ − naïve CD4+ T cells were activated with anti-CD3 and anti-CD28 and cultured with IL-4 + anti-IFN-γ (Th2 conditions). After 5 days of differentiation cells were collected and counted. Differentiated cells (1x106) were then re-stimulated with anti-CD3 (4 μg/ml) for 24 hours. Cell-free supernatant was collected and tested for various cytokines using ELISA. Data are the mean ± s.d. of results from two mice and representative of more than 5 experiments. Students t test was performed to calculate p values.
(D) Cells activated and cultured as in A were re-stimulated with anti-CD3 for 5 hours. Following stimulation cells were stained with anti-IL-4 and anti-IL-9. Numbers in flow cytometry dot plots indicate the percentages of cells in the quadrant.
(E) After re-stimulation of differentiated Th2 cells with anti-CD3 and recovery of supernatants as described in (A), cell pellets were collected, RNA was isolated, and quantitative PCR was performed for the indicated cytokines (mean ± s.d.). Data are representative of two experiments.