Abstract
Both humoral and cellular immune responses are impaired in aged individuals, leading to decreased vaccine responses. Although T cell defects occur, defects in B cells play a significant role in age-related humoral immune changes. The ability to undergo class switch recombination (CSR), the enzyme for CSR, AID (activation-induced cytidine deaminase) and the transcription factor E47 are all decreased in aged stimulated B cells. We here present an overview of age-related changes in human B cell markers and functions, and also discuss some controversies in the field of B cell aging.
Keywords: Aging, B lymphocytes, immunoglobulin class switch, influenza vaccination
1. Introduction
Aging affects the humoral immune response both quantitatively and qualitatively, as specificity and class of antibody produced are changed (Frasca and Blomberg, 2009; Linton and Dorshkind, 2004). The changes in the humoral immune response during aging significantly contribute to the increased susceptibility of the elderly to infectious diseases and reduce the protective effects of vaccination (McElhaney and Effros, 2009). Not only decreased antibody production but also reduced duration of protective immunity following immunization has been reported (Steger et al., 1996).
High-affinity protective antibodies are produced in the germinal centers (GC) of secondary lymphoid tissue during affinity maturation processes which are characterized by somatic hypermutation (SHM) of immunoglobulin (Ig) genes and subsequent selection of the genes encoding the best antibodies (Klein and Dalla-Favera, 2008; Longerich et al., 2006).
The effects of age on antibody affinity maturation are controversial and results obtained by different groups are conflicting. Increased level of mutations in Ig genes have been reported in elderly individuals (Dunn-Walters et al., 1997; Kolar et al., 2006), and attributed to accumulation rather than altered rate, as SHM occurs at the same rate in young and elderly individuals (Banerjee et al., 2002). However, the same group has also shown that B cell repertoire as measured by spectratyping and DNA sequencing in individuals aged 86-94 has less Ig diversity than young especially in the more frail group (Gibson et al., 2009). Another study has shown that young individuals have more blood lymphocytes with mutated clones, as compared with those from elderly individuals; however, among the mutated clones, the frequency, location, and types of substitutions were similar between the young and the old groups (Radl et al., 1975).
The decreased ability of aged individuals to produce high affinity protective antibody responses against infectious agents results at least partially from defects in T cells, such as reduction in naïve T cells and a concomitant increase in memory/effector T cells (Pawelec et al., 2002), loss in CD28 expression (Vallejo, 2005), and is associated with an increase in cytomegalovirus (CMV) positivity (Grubeck-Loebenstein et al., 2009; Pawelec et al., 2009). Cytokine production and T-cell proliferation are also affected with age (Pawelec et al., 2002), as a consequence of signal transduction defects due to both lipid raft formation and intracellular effectors (Larbi et al., 2008; Sadighi Akha and Miller, 2005).
Although B cell function may suffer from lack of optimal T cell help in aging, intrinsic changes in B cells also occur and have a significant impact on antibody production. By intrinsic changes we mean B cell functions not requiring external cellular (such as T cell) signals. These intrinsic changes, as already shown in murine B cells (Frasca et al., 2004), include decreases in the E2A-encoded transcription factor E47, activation-induced cytidine deaminase (AID), and class switch recombination (CSR) measured by IgG production in culture supernatants and circle transcripts (CTs) in human B cells, in response to both polyclonal (Frasca et al., 2008) and influenza-specific (Frasca et al., submitted) stimulation. Objectives of our work are to establish biomarkers of human B cell function and associate these with a biologically relevant immune response, such as the influenza vaccine. Our results clearly indicate that AID can accurately track optimal immune responses and activity, and can be a valid predictor of vaccine effectiveness in humans.
2. Age-related changes in B cell markers
The absolute numbers of human B cell precursors in the bone marrow have been shown to decline moderately (McKenna et al., 2001) or not (Rossi et al., 2003) with age. However, the numbers of mature human B cells significantly decrease with age, as us (Frasca et al., 2008) and others (Chong et al., 2005; Franceschi et al., 1995; Paganelli et al., 1992; Shi et al., 2005) have shown.
In our initial study (Frasca et al., 2008), we have analyzed the composition of the peripheral B cell pool in individuals of different ages (18-86 years). Briefly, we determined the percentages and the absolute numbers of total CD19+ B cells, as well as the percentages and absolute numbers of naive (IgG−/IgA−/CD27−), IgM memory (IgG−/IgA−/CD27+), and total switch memory (IgG+/IgA+) B cells. It has been shown (Tangye and Good, 2007) that in the IgG+/IgA+ B cell subset, not only CD27+ cells but also CD27− cells express mutated IgV region genes, high levels of the costimulatory molecules CD80 and CD86, and high in vitro Ig secretion as compared with naive B cells and therefore can be considered as switch memory B cells. We have found that both the IgG+/IgA+/CD27− as well as IgG+/IgA+/CD27+ cells decrease with age (unpublished results and below). Our results showed that both the percentages and the numbers of total CD19+ B cells decrease with age. The percentage of naive B cells increases with age, but the number was found not significantly different in young and elderly subjects. Similarly, in the human tonsil, naive B cells have been shown to increase with age (Kolar et al., 2006). The percentage of IgM memory B cells are not statistically different between young and elderly subjects, but the absolute number was decreased (Frasca et al., 2008). The reduction in IgM cells has been suggested to cause reduced specific antibody titers in elderly individuals vaccinated against pneumococcal polysaccharides and to Streptococcus pneumoniae infection (Shi et al., 2005). Total switch memory B cells decrease in both percentage and number with age. The significant decrease in switch memory B cells and the increase in the percentage of naive and IgM memory B cells suggest an intrinsic defect in the ability of old B cells to undergo CSR. The results on age-related changes in naïve, IgM memory and switch memory B cells have been obtained by staining peripheral blood-derived (Ficoll PBMC) B cells. There is one other report (Colonna-Romano et al., 2003) and a review (Siegrist and Aspinall, 2009) showing that memory B cell percentages increase not significantly with age, but the majority of reports favor a decrease (Chong et al., 2005; Frasca et al., 2008; Shi et al., 2005).
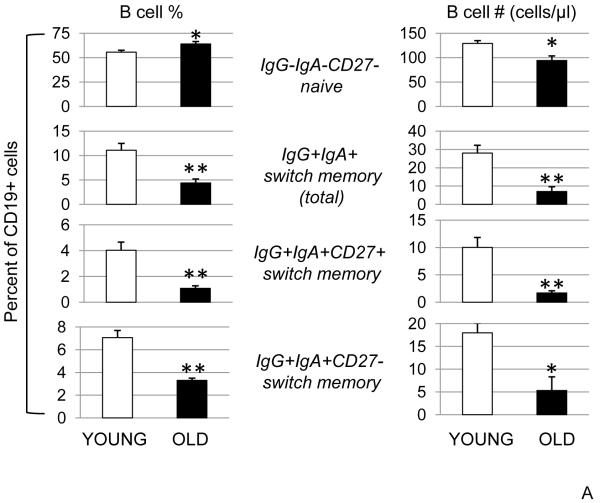
More recently, we have determined the percentages and the absolute numbers of total CD19+ B cells and B cell subsets (naïve, IgM memory, switch memory) by staining 100 μl of blood from donors of different ages (20-90 years). The results obtained with whole blood staining show comparable age-related effects with the results obtained with peripheral blood-derived (Ficoll PBMC) B cell staining. However, the relative percentages of the B cell subsets differed. Results in Fig. 1A show the age-related changes in naïve and switch memory B cells from 112 individuals (66 young and 46 elderly), as evaluated by blood staining. We found that naïve B cell numbers but not percentages, and total switch memory (both CD27+ and CD27−) B cell percentages and numbers were significantly decreased by age. The percentages of IgM memory B cells were unchanged by age but the absolute numbers were significantly decreased (not shown). In the literature others have found an increase in all memory B cells, but this was not significant (Colonna-Romano et al., 2003). Our results below clearly indicate that not only the numbers of switch memory B cells decrease with age but also the function of class switching B cells. This finding is significant especially for specific responses, i.e. anti-influenza vaccine response.
Figure 1. Age-related changes in the percentages and numbers of B cell subsets.
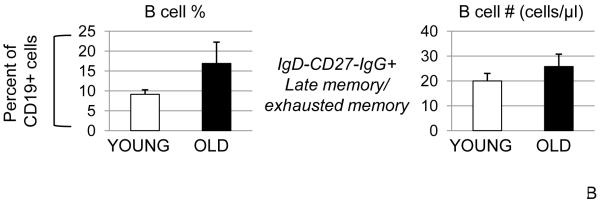
A. One hundred μl of blood from young (20-64 years) and elderly (≥65 years) subjects were stained to evaluate the percentages of naïve and switch memory B cells. The percentages of IgM memory (IgG−IgA−CD27+, not shown) were unchanged in young and old and together with naïve and total switch memory add up to 100%. Sixty-six young and forty-six elderly subjects were evaluated. The differences between young and elderly subjects were evaluated by the Wilcoxon test (two-tailed). p<0.05 (*), p<0.01 (**). B. Fifty young and twenty-five elderly subjects were evaluated.
We have extended our analysis to another subpopulation of B cells: late memory/exhausted memory B cells. These memory B cells have been defined as memory B cells which have down-regulated the CD27 marker (CD19+IgD−CD27−), most of them are IgG+, they carry short telomeres (therefore non functional), and have been reported to be increased with age (Colonna-Romano et al., 2009). Results in Fig. 1B show that late memory/exhausted memory B cell percentages are increased in the elderly, but the numbers are comparable in young and elderly subjects. Our percentages are comparable to those from Colonna-Romano et al. (2009), but we have observed more variability in the elderly and therefore the differences are not significant. These cells could be a subset of IgG+CD27− switch memory which are IgD−, or they could be cells transitioning from naïve or transitional to these CD27− IgG+ memory cells. Regardless, they are likely non functional/exhausted and therefore less/non relevant for an in vivo antibody response such as specific anti-influenza vaccine response. The memory B cells responsible for driving the rapid secondary antibody response after re-exposure to the antigen, which is important for the elimination of the pathogens not cleared by pre-existing antibodies, are CD19+Ig+CD27+ (Crotty et al., 2004) which would include the switch memory (IgG+IgA+CD27+) and the IgM memory (IgG−IgA−CD27+). These cells are long-lived and quiescent, they express somatically hypermutated Ig V genes and are able to generate more rapid and robust responses compared to the antigen-inexperienced naïve B cells. Epidemiological data from the pandemic H1N1 infection indicate that the currently circulating H1N1 strain mainly affects people younger than 60 years of age, suggesting that cross-reactive, effector memory B cells similar to those described by Crotty et al. are present in older individuals helping them to control the infection (Castellino et al., 2009). Most likely the first “memory” B lineage cell to see the previously encountered pathogen would be a long-lived plasma cell in the bone marrow and/or its antibody. These, together with the CD27+ memory B cells would provide the memory response, but our results (Frasca et al., 2008) indicate that a new response generated in the elderly as a result of stimulating naïve and IgM memory is suboptimal. These age-related changes in B cell markers are summarized in Table 1.
Table 1.
Age-related changes in B cell markers
| CELLS | CHANGES WITH AGE (≥65 yrs) | REFERENCES |
|---|---|---|
| Total B cells (CD19+) | Decreased % and # |
Paganelli et al., 1992
Franceschi et al., 1995 Chong et al., 2005 Shi et al., 2005 Frasca et al., 2008 |
| Naïve* | Increased %, unchanged or decreased # |
Chong et al., 2005
Kolar et al., 2006 Shi et al., 2005 Frasca et al., 2008 |
| Memory (CD27+) | Increased % (not significant) | Colonna-Romano et al., 2003 |
| IgM memory (IgG-IgA-CD27+) |
Unchanged %, decreased # |
Shi et al., 2005
Frasca et al., 2008 |
| Switch memory (total)* | Decreased % and # |
Chong et al., 2005
Shi et al., 2005 Frasca et al., 2008 |
| Late/exhausted memory* |
Increased % | Colonna-Romano et al., 2009 |
| CD19+CD62L+ | Decreased % | De Martinis et al., 2000 |
| CD19+CD49d/50+ | Decreased % | De Martinis et al., 2000 |
| CD19+CD38+ | Decreased % | Radl et al., 1975 |
| CD19+CD21+ | Decreased % | Radl et al., 1975 |
| CD19+CD40+ | Unchanged | Colonna-Romano et al., 2003 |
Designations from Fig. 1. All subsets are CD19+. CD markers are described in the text.
Data from other laboratories (Table 1) have shown that the absolute numbers of B cells expressing the homing receptor CD62L, the adhesion molecules CD49d and CD50 (involved in leukocyte-endothelial cell interactions) were found to be decreased in elderly compared to young subjects (De Martinis et al., 2000), indicating that the ability of B cells from old individuals to adhere to the endothelium may be decreased. The absolute numbers of B cells expressing CD38 (a GC B cell marker) and CD21 (marginal zone B cell marker) in the tonsils are reduced by age (Radl et al., 1975), suggesting an impairment in the first line of defense against bacterial antigens in the elderly.
As for costimulatory molecules, we have evaluated the levels of expression of CD80, CD86 and CD40 on B cells from the same individuals of different ages as above, by whole blood staining. CD80, CD86 and CD40 are similarly expressed on B cells from young and elderly individuals (data not shown). Others have also shown that there are no changes in CD40 expression by B cells (Colonna-Romano et al., 2003).
3. Age-related changes in B cell antibody production
At least one contributing factor in B cells from old individuals responsible for their inability to respond well to vaccination is a defect in the molecular events leading to the production of secondary isotypes, known as CSR (Frasca et al., submitted; and results herein). Specific antibody responses in humans immunized with vaccines against tetanus toxin, encephalitis viruses, Salmonella, or pneumococcus decrease with age (LeMaoult et al., 1997). The IgG response to an influenza vaccine is also decreased in the elderly (>65 years of age) (Gardner et al., 2001; Murasko et al., 2002).
During an immune response, B cells can switch the expression of surface Ig from IgM to IgG, IgE, or IgA. This DNA recombination takes place between two switch (S) regions, one upstream (5′) of the μ CH and one 5′ of one of the other CH regions (γ, ε or α) to produce IgG, IgE, or IgA, respectively. The class switch recombination (CSR) requires chromatin opening of S regions, recognition and cleavage of the target DNA by an endonuclease, and repair and ligation of the cleaved ends (Casellas et al., 1998; Honjo et al., 2002; Kaminski and Stavnezer, 2004; Manis et al., 1998). CSR is extremely important for the humoral immune response, because it generates antibodies of the same specificity but with different effector functions. Patients who cannot switch their Ig class have been described. These include those with hyper-IgM (HIGM), due to a genetic defect in CD40L on T cells required to trigger CD40+ B cells. These patients (HIGM1) are not only prone to bacterial and enteroviral infections, in a similar way to other patients with severe B-cell deficiencies, but also to opportunistic infections mainly due to Pneumocystis carinii and Cryptosporidium as observed in patients with T-cell defects and to neutropenic complications (Levy et al., 1997; Lougaris et al., 2005). Other HIGM patients (HIGM2) have a loss in AID, critical for CSR and somatic hypermutation (SHM). They present not only a CSR defect characterized by a lack of IgG, IgA and IgE production but also defective generation of SHM in the Ig variable region genes resulting in impaired antibody affinity maturation (Durandy, 2002). These patients show upper and lower respiratory tract infection, otitis, diarrhea, oral ulcers, and autoimmunity and fail to respond to vaccination (Durandy, 2002; Notarangelo et al., 1992).
We have previously shown in mice that the transcription factor E47, which regulates AID and Ig class switch, is down-regulated in aged murine B cells due to decreased mRNA stability (Frasca et al., 2005; Frasca et al., 2004). The transcription factor E47 is necessary for CSR because it transcriptionally regulates Aicda, the gene encoding AID (Quong et al., 2002; Sayegh et al., 2003). E47 is a class I basic helix loop helix protein, able to bind with relatively high affinity to the palindromic DNA sequence CANNTG, referred to as an E-box site (Ephrussi et al., 1985; Henthorn et al., 1990; Quong et al., 2002). E-boxes have been found in the promoter and enhancer regions of many B lineage-specific genes and regulate a large number of processes involved in B cell commitment and differentiation (Kee et al., 2002; Massari and Murre, 2000; Murre et al., 1989; Schlissel et al., 1991; Sigvardsson et al., 1997). In our experiments, we have described an intrinsic defect in B cells from old mice, as we used purified B cells to avoid potential contribution/confusion generated from aged T cells, also known to be defective, during Ig class switch in vitro.
4. Molecular mechanisms for the reduced antibody production by human B cells
In humans, we have investigated whether aging also affects E47, AID and CSR in B cells isolated from the peripheral blood of individuals of different ages (20-90 years). In our preliminary study (Frasca et al., 2008), we have shown that anti-CD40/IL-4-stimulated CD19+ B cells have greatly reduced (almost 4-fold) their ability to undergo in vitro class switch with age and we have shown that there is an intrinsic defect in the expression of E47, AID and IgG. A lower amount of IgG in vivo should lead to reduced protection against a new response, e.g. influenza vaccine. Although in our study we saw a clear and significant decrease in both E47, AID with age, there is also an increase in the variability in the aged population for these two biomarkers. In our experiments, E47 and AID were significantly correlated (r=0.80, p<0.01). Moreover, AID levels were also correlated with CTs, the final products of CSR (r=0.79, p<0.01).
To investigate whether the intrinsic defect we saw in class switch depends on an intrinsic defect in the subsets of memory B cells or from the reduction in the numbers of memory B cells, we sorted naïve (CD19+CD27−) and memory (CD19+CD27+) B cells which were then stimulated in vitro by anti-CD40/IL-4 and F(ab’)2 fragments of anti-human IgM, used as surrogate antigen, because naïve B cells require the activation of the BCR signal transduction to undergo class switch, whereas memory B cells do not (Bernasconi et al., 2002). Our results demonstrated that both memory and naïve B cells showed intrinsic defects in class switch with age (Frasca et al., 2008). The majority (90%) of the in vitro switched response from memory cells comes from the IgM memory cells (CD19+CD27+IgG-IgA−) (Frasca et al., 2008), whereas most switch memory cells (75%) do not respond/express AID when restimulated. Therefore our results on reduced CSR with age are not only due to an age-related reduction in the absolute numbers of naïve (30%) and IgM memory (50%) B cells, which are 80-85% of all B cells, (Frasca et al., 2008), but also to an intrinsic defect in the ability of these cells to undergo CSR.
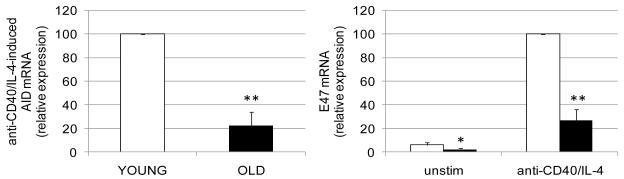
AID mRNA expression was significantly lower (about 5-fold) in stimulated B cells from elderly as compared with young subjects, in response to anti-CD40/IL-4, (Frasca et al., 2008) (Fig. 2, left). Similar results have been recently obtained with CpG (Frasca et al., submitted).
Figure 2. Aging decreases AID and E47 mRNA expression, E47 mRNA stability and increases TTP.
B cells (106 cells/ml) were cultured with anti-CD40/IL-4, for 1 day (E47) or 7 days (AID), or left unstimulated. At the end of these times, cells were harvested, mRNA extracted and qPCR performed. Thirty young and thirty elderly subjects were evaluated. White columns: young; black columns, elderly. The differences between young and elderly subjects were evaluated by two-tailed Student’s t test. p<0.05 (*), p<0.01 (**).
AID is transcriptionally regulated by the E2A-endoded transcription factor E47 (Sayegh et al., 2003). Our studies on murine B cells have characterized the mechanisms for the age-related decrease in E47 levels in old splenic B cells, which is due to mRNA instability (Frasca et al., 2007; Frasca et al., 2005), whereas E47 protein degradation rates are comparable in young versus aged B cells. The stability of E47 mRNA is regulated at least in part by the p38 MAPK signal transduction cascade, which phosphorylates the protein, tristetraprolin (TTP), that interacts with the adenylate/uridylate-rich elements (ARE) in the 3′ untranslated region (UTR) of many mRNAs decreasing their stability (Lai et al., 2006; Stoecklin and Anderson, 2006). We have found that tristetraprolin (TTP), a physiological regulator of mRNA expression and stability, is involved in the degradation of the E47 mRNA in splenic B cells from old mice (Frasca et al., 2007).
E47 mRNA expression is also significantly lower in B cells from elderly as compared to those from young subjects. Unstimulated B cells express barely detectable levels of E47 mRNA but here too, levels of E47 in elderly are significantly lower than in young B cells. Stimulation of purified B cells from young and elderly subjects with anti-CD40/IL-4 induced a marked increase in E47 mRNA expression, but the levels of mRNA in elderly are significantly lower than those seen in young subjects (Fig. 2, right) (Frasca et al., 2008). We have preliminary results (data not shown) indicating that E47 mRNA levels in human stimulated CD19+ B cells from young and old subjects are regulated, at least in part, by mRNA stability and TTP, as we have previously shown in mice (Frasca et al., 2005; Frasca et al., 2007). Therefore, B cells from human elderly individuals show the same intrinsic defects of B cells from old mice. As a consequence, they are impaired in their ability to undergo in vitro class switch. These results are the first to identify molecular mechanisms responsible for the reduced antibody production by B cells from elderly individuals. We are currently also exploring other possible mechanisms for the increased E47 mRNA degradation in aged B cells, including miRNA analyses.
5. Age-related decrease in the response to influenza vaccination
The inability of B cells from elderly individuals to respond to vaccination is likely due to defects both in T cell help to B cells and in the molecular pathways in B cells leading to the production of secondary isotypes by CSR. We recently began to investigate whether AID can be used as a biomarker for specific in vivo B cell responses. We initiated a series of experiments to measure the antibody response to seasonal influenza vaccination by hemagglutination inhibition assay (HI) and associated this with the B cell response (AID) to the vaccine in vitro. Infectious pathogenic diseases are fairly common, with influenza alone affecting up to an estimated 50 million people each year in the U.S.A. Approximately 40,000 deaths are attributed to influenza, with most of the affected being the elderly and those patients with compromised immune systems. The serologic response to influenza virus vaccine varies with age (de Bruijn et al., 1999; Goodwin et al., 2006; McElhaney, 2008; McMurry et al., 2008). Successive annual vaccinations increase protection against influenza (Ahmed et al., 1995; Keitel et al., 1988; Thompson et al., 2004), suggesting that cellular and humoral immune mechanisms are important for protection in elderly individuals. Antibody responses to influenza vaccination have previously been correlated with T cell function (Goronzy et al., 2001; Saurwein-Teissl et al., 2002).
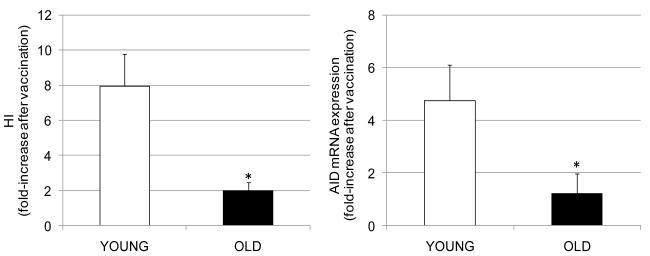
In our study, we analyzed the serum HI response in 29 young and 8 elderly, before (t0) or one month after influenza vaccination (t28), to evaluate antibody production to the seasonal influenza vaccine for the 2008-2009 and 2009-2010 influenza seasons (Frasca et al., submitted; Blomberg, Frasca, Diaz, 2010). The HI assay is based on the ability of certain viruses or viral components to hemagglutinate the red blood cells of specific animal species (Hsiung et al., 1994). Antibodies specific to influenza can inhibit this agglutination. The HI test is useful for the measurement of antibody titers of sera and is the most established correlate with vaccine protectiveness (Sambhara and McElhaney, 2009). The protective level of specific IgG produced in response to the influenza vaccine is an HI titer of 1:40 (Sambhara and McElhaney, 2009; Wood et al., 1977). In our studies, B cells from the same individuals of different ages as above were stimulated in vitro with the influenza vaccine to induce AID mRNA expression, before (t0), or after (t28) influenza vaccination, to induce optimal AID mRNA. Our results (Fig. 3) show that aging similarly decreases the influenza-specific serum HI response, and the in vitro AID mRNA expression. B cell function, as measured by AID in stimulated B cells, can predict the ability to generate an optimal influenza vaccine response. Therefore, AID is an effective tool to assess vaccine responses and B cell function.
Figure 3. The response to the seasonal influenza vaccine decreases with age.
Sera were collected from 37 subjects of different ages (29 young and 8 elderly) at t0 (before vaccination) and at t28 (one month after vaccination) and analyzed by HI to evaluate antibody production to vaccine (left). B cells were isolated from the peripheral blood of the same subjects at t0 and t28 and cultured for 7 days with the influenza vaccine with which they were immunized (right). Data are from the 2008-2009 and 2009-2010 influenza seasons. Results are expressed as fold-increase after vaccination. calculated as follows: values after vaccination/values before vaccination. White columns: young; black columns, elderly.The differences between young and elderly subjects were significant at p<0.05, as evaluated by the Wilcoxon test (two-tailed).
6. Conclusions
In conclusion, our results show an intrinsic defect in the ability of B cells from individuals 65 years of age and older to undergo CSR. The transcription factor E47 in activated B cells is significantly impaired by aging, due to decreased mRNA stability. This leads to a reduction in AID and, in turn, to less switched antibodies produced by the activated B cells. Moreover, we have identified new biomarkers for an optimal vaccine response in humans. AID in influenza-stimulated B cells correlates with the serum HI response. These results offer targets for diagnostic as well as potential therapeutic treatment to improve the humoral immune response in the elderly as well as other immune compromised individuals and should be highly significant and impactful for the field of human vaccine response.
Acknowledgements
We thank doctors (in particular Dr. R. Schwartz, chairman, and Dr. J. Ryan) and nurses of the Family Medicine Department and Sandra Chen, Employee Health Manager, at the University of Miami Miller School of Medicine. We thank Jim Phillips and the Sylvester Comprehensive Cancer Center Flow Cytometry Core Resource, and Michelle Perez for secretarial assistance.
This work was supported by NIH AG-17618, AG-23717 and AG-28586 (BBB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed AE, Nicholson KG, Nguyen-Van-Tam JS. Reduction in mortality associated with influenza vaccine during 1989-90 epidemic. Lancet. 1995;346:591–595. doi: 10.1016/s0140-6736(95)91434-x. [DOI] [PubMed] [Google Scholar]
- Banerjee M, Mehr R, Belelovsky A, Spencer J, Dunn-Walters DK. Age- and tissue-specific differences in human germinal center B cell selection revealed by analysis of IgVH gene hypermutation and lineage trees. Eur J Immunol. 2002;32:1947–1957. doi: 10.1002/1521-4141(200207)32:7<1947::AID-IMMU1947>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- Blomberg BB, Frasca D, Diaz M. AID in aging and autoimmune disease. In: Diaz M, Fugmann S, Papavasiliou N, editors. DNA Deamination and the Immune System. Imperial College Press; London: 2010. 2010 in press. [Google Scholar]
- Casellas R, Nussenzweig A, Wuerffel R, Pelanda R, Reichlin A, Suh H, Qin XF, Besmer E, Kenter A, Rajewsky K, Nussenzweig MC. Ku80 is required for immunoglobulin isotype switching. Embo J. 1998;17:2404–2411. doi: 10.1093/emboj/17.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino F, Galli G, Del Giudice G, Rappuoli R. Generating memory with vaccination. Eur J Immunol. 2009;39:2100–2105. doi: 10.1002/eji.200939550. [DOI] [PubMed] [Google Scholar]
- Chong Y, Ikematsu H, Yamaji K, Nishimura M, Nabeshima S, Kashiwagi S, Hayashi J. CD27(+) (memory) B cell decrease and apoptosis-resistant CD27(−) (naive) B cell increase in aged humans: implications for age-related peripheral B cell developmental disturbances. Int Immunol. 2005;17:383–390. doi: 10.1093/intimm/dxh218. [DOI] [PubMed] [Google Scholar]
- Colonna-Romano G, Bulati M, Aquino A, Pellicano M, Vitello S, Lio D, Candore G, Caruso C. A double-negative (IgD−CD27−) B cell population is increased in the peripheral blood of elderly people. Mech Ageing Dev. 2009;130:681–690. doi: 10.1016/j.mad.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Colonna-Romano G, Bulati M, Aquino A, Scialabba G, Candore G, Lio D, Motta M, Malaguarnera M, Caruso C. B cells in the aged: CD27, CD5, and CD40 expression. Mech Ageing Dev. 2003;124:389–393. doi: 10.1016/s0047-6374(03)00013-7. [DOI] [PubMed] [Google Scholar]
- Crotty S, Aubert DA, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- de Bruijn IA, Remarque EJ, Jol-van der Zijde CM, van Tol MJ, Westendorp RG, Knook DL. Quality and quantity of the humoral immune response in healthy elderly and young subjects after annually repeated influenza vaccination. J Infect Dis. 1999;179:31–36. doi: 10.1086/314540. [DOI] [PubMed] [Google Scholar]
- De Martinis M, Modesti M, Loreto MF, Quaglino D, Ginaldi L. Adhesion molecules on peripheral blood lymphocyte subpopulations in the elderly. Life Sci. 2000;68:139–151. doi: 10.1016/s0024-3205(00)00924-3. [DOI] [PubMed] [Google Scholar]
- Dunn-Walters DK, Boursier L, Spencer J. Hypermutation, diversity and dissemination of human intestinal lamina propria plasma cells. Eur J Immunol. 1997;27:2959–2964. doi: 10.1002/eji.1830271131. [DOI] [PubMed] [Google Scholar]
- Durandy A. Hyper-IgM syndromes: a model for studying the regulation of class switch recombination and somatic hypermutation generation. Biochem Soc Trans. 2002;30:815–818. doi: 10.1042/bst0300815. [DOI] [PubMed] [Google Scholar]
- Ephrussi A, Church GM, Tonegawa S, Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985;227:134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Monti D, Sansoni P, Cossarizza A. The immunology of exceptional individuals: the lesson of centenarians. Immunol Today. 1995;16:12–16. doi: 10.1016/0167-5699(95)80064-6. [DOI] [PubMed] [Google Scholar]
- Frasca D, Diaz A, Romero M, Landin AM, Phillips M, Lechner S, Ryan JG, Blomberg BB. Intrinsic defects in B cell response to seasonal influenza vaccination in elderly humans. Vaccine. 2010 doi: 10.1016/j.vaccine.2010.10.023. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Blomberg BB. Effects of aging on B cell function. Current opinion in immunology. 2009;21:425–430. doi: 10.1016/j.coi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Landin AM, Alvarez JP, Blackshear PJ, Riley RL, Blomberg BB. Tristetraprolin, a negative regulator of mRNA stability, is increased in old B cells and is involved in the degradation of E47 mRNA. J Immunol. 2007;179:918–927. doi: 10.4049/jimmunol.179.2.918. [DOI] [PubMed] [Google Scholar]
- Frasca D, Landin AM, Lechner SC, Ryan JG, Schwartz R, Riley RL, Blomberg BB. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol. 2008;180:5283–5290. doi: 10.4049/jimmunol.180.8.5283. [DOI] [PubMed] [Google Scholar]
- Frasca D, Van der Put E, Landin AM, Gong D, Riley RL, Blomberg BB. RNA stability of the E2A-encoded transcription factor E47 is lower in splenic activated B cells from aged mice. J Immunol. 2005;175:6633–6644. doi: 10.4049/jimmunol.175.10.6633. [DOI] [PubMed] [Google Scholar]
- Frasca D, Van der Put E, Riley RL, Blomberg BB. Reduced Ig class switch in aged mice correlates with decreased E47 and activation-induced cytidine deaminase. J Immunol. 2004;172:2155–2162. doi: 10.4049/jimmunol.172.4.2155. [DOI] [PubMed] [Google Scholar]
- Gardner EM, Bernstein ED, Dran S, Munk G, Gross P, Abrutyn E, Murasko DM. Characterization of antibody responses to annual influenza vaccination over four years in a healthy elderly population. Vaccine. 2001;19:4610–4617. doi: 10.1016/s0264-410x(01)00246-8. [DOI] [PubMed] [Google Scholar]
- Gibson KL, Wu YC, Barnett Y, Duggan O, Vaughan R, Kondeatis E, Nilsson BO, Wikby A, Kipling D, Dunn-Walters DK. B-cell diversity decreases in old age and is correlated with poor health status. Aging cell. 2009;8:18–25. doi: 10.1111/j.1474-9726.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75:12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B, Della Bella S, Iorio AM, Michel JP, Pawelec G, Solana R. Immunosenescence and vaccine failure in the elderly. Aging Clin Exp Res. 2009;21:201–209. doi: 10.1007/BF03324904. [DOI] [PubMed] [Google Scholar]
- Henthorn P, Kiledjian M, Kadesch T. Two distinct transcription factors that bind the immunoglobulin enhancer microE5/kappa 2 motif. Science. 1990;247:467–470. doi: 10.1126/science.2105528. [DOI] [PubMed] [Google Scholar]
- Honjo T, Kinoshita K, Muramatsu M. Molecular mechanism of class switch recombination: linkage with somatic hypermutation. Annu Rev Immunol. 2002;20:165–196. doi: 10.1146/annurev.immunol.20.090501.112049. [DOI] [PubMed] [Google Scholar]
- Hsiung GD, Fong CKY, Landry ML. Diagnostic Virology. 4 ed. Yale University Press; 1994. [Google Scholar]
- Kaminski DA, Stavnezer J. Antibody class switching: uncoupling S region accessibility from transcription. Trends Genet. 2004;20:337–340. doi: 10.1016/j.tig.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Kee BL, Bain G, Murre C. IL-7Ralpha and E47: independent pathways required for development of multipotent lymphoid progenitors. Embo J. 2002;21:103–113. doi: 10.1093/emboj/21.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitel WA, Cate TR, Couch RB. Efficacy of sequential annual vaccination with inactivated influenza virus vaccine. Am J Epidemiol. 1988;127:353–364. doi: 10.1093/oxfordjournals.aje.a114809. [DOI] [PubMed] [Google Scholar]
- Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nature reviews. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- Kolar GR, Mehta D, Wilson PC, Capra JD. Diversity of the Ig repertoire is maintained with age in spite of reduced germinal centre cells in human tonsil lymphoid tissue. Scand J Immunol. 2006;64:314–324. doi: 10.1111/j.1365-3083.2006.01817.x. [DOI] [PubMed] [Google Scholar]
- Lai WS, Parker JS, Grissom SF, Stumpo DJ, Blackshear PJ. Novel mRNA Targets for Tristetraprolin (TTP) Identified by Global Analysis of Stabilized Transcripts in TTP-Deficient Fibroblasts. Mol Cell Biol. 2006;26:9196–9208. doi: 10.1128/MCB.00945-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larbi A, Franceschi C, Mazzatti D, Solana R, Wikby A, Pawelec G. Aging of the immune system as a prognostic factor for human longevity. Physiology (Bethesda) 2008;23:64–74. doi: 10.1152/physiol.00040.2007. [DOI] [PubMed] [Google Scholar]
- LeMaoult J, Szabo P, Weksler ME. Effect of age on humoral immunity, selection of the B-cell repertoire and B-cell development. Immunol Rev. 1997;160:115–126. doi: 10.1111/j.1600-065x.1997.tb01032.x. [DOI] [PubMed] [Google Scholar]
- Levy J, Espanol-Boren T, Thomas C, Fischer A, Tovo P, Bordigoni P, Resnick I, Fasth A, Baer M, Gomez L, Sanders EA, Tabone MD, Plantaz D, Etzioni A, Monafo V, Abinun M, Hammarstrom L, Abrahamsen T, Jones A, Finn A, Klemola T, DeVries E, Sanal O, Peitsch MC, Notarangelo LD. Clinical spectrum of X-linked hyper-IgM syndrome. The Journal of pediatrics. 1997;131:47–54. doi: 10.1016/s0022-3476(97)70123-9. [DOI] [PubMed] [Google Scholar]
- Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- Longerich S, Basu U, Alt F, Storb U. AID in somatic hypermutation and class switch recombination. Current opinion in immunology. 2006;18:164–174. doi: 10.1016/j.coi.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Lougaris V, Badolato R, Ferrari S, Plebani A. Hyper immunoglobulin M syndrome due to CD40 deficiency: clinical, molecular, and immunological features. Immunol Rev. 2005;203:48–66. doi: 10.1111/j.0105-2896.2005.00229.x. [DOI] [PubMed] [Google Scholar]
- Manis JP, Gu Y, Lansford R, Sonoda E, Ferrini R, Davidson L, Rajewsky K, Alt FW. Ku70 is required for late B cell development and immunoglobulin heavy chain class switching. J Exp Med. 1998;187:2081–2089. doi: 10.1084/jem.187.12.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhaney JE. Influenza vaccination in the elderly: seeking new correlates of protection and improved vaccines. Aging health. 2008;4:603–613. doi: 10.2217/1745509X.4.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Current opinion in immunology. 2009;21:418–424. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna RW, Washington LT, Aquino DB, Picker LJ, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) in 662 consecutive bone marrow specimens by 4-color flow cytometry. Blood. 2001;98:2498–2507. doi: 10.1182/blood.v98.8.2498. [DOI] [PubMed] [Google Scholar]
- McMurry JA, Johansson BE, De Groot AS. A call to cellular & humoral arms: enlisting cognate T cell help to develop broad-spectrum vaccines against influenza A. Hum Vaccin. 2008;4:148–157. doi: 10.4161/hv.4.2.5169. [DOI] [PubMed] [Google Scholar]
- Murasko DM, Bernstein ED, Gardner EM, Gross P, Munk G, Dran S, Abrutyn E. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol. 2002;37:427–439. doi: 10.1016/s0531-5565(01)00210-8. [DOI] [PubMed] [Google Scholar]
- Murre C, McCaw PS, Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989;56:777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Notarangelo LD, Duse M, Ugazio AG. Immunodeficiency with hyper-IgM (HIM) Immunodefic Rev. 1992;3:101–121. [PubMed] [Google Scholar]
- Paganelli R, Quinti I, Fagiolo U, Cossarizza A, Ortolani C, Guerra E, Sansoni P, Pucillo LP, Scala E, Cozzi E, et al. Changes in circulating B cells and immunoglobulin classes and subclasses in a healthy aged population. Clinical and experimental immunology. 1992;90:351–354. doi: 10.1111/j.1365-2249.1992.tb07954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G, Barnett Y, Forsey R, Frasca D, Globerson A, McLeod J, Caruso C, Franceschi C, Fulop T, Gupta S, Mariani E, Mocchegiani E, Solana R. T cells and aging, January 2002 update. Front Biosci. 2002;7:d1056–1183. doi: 10.2741/a831. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol. 2009;19:47–56. doi: 10.1002/rmv.598. [DOI] [PubMed] [Google Scholar]
- Quong MW, Romanow WJ, Murre C. E protein function in lymphocyte development. Annu Rev Immunol. 2002;20:301–322. doi: 10.1146/annurev.immunol.20.092501.162048. [DOI] [PubMed] [Google Scholar]
- Radl J, Sepers JM, Skvaril F, Morell A, Hijmans W. Immunoglobulin patterns in humans over 95 years of age. Clinical and experimental immunology. 1975;22:84–90. [PMC free article] [PubMed] [Google Scholar]
- Rossi MI, Yokota T, Medina KL, Garrett KP, Comp PC, Schipul AH, Jr., Kincade PW. B lymphopoiesis is active throughout human life, but there are developmental age-related changes. Blood. 2003;101:576–584. doi: 10.1182/blood-2002-03-0896. [DOI] [PubMed] [Google Scholar]
- Sadighi Akha AA, Miller RA. Signal transduction in the aging immune system. Current opinion in immunology. 2005;17:486–491. doi: 10.1016/j.coi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Sambhara S, McElhaney JE. Immunosenescence and influenza vaccine efficacy. Curr Top Microbiol Immunol. 2009;333:413–429. doi: 10.1007/978-3-540-92165-3_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I, Parson W, Bock G, Schonitzer D, Trannoy E, Grubeck-Loebenstein B. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- Sayegh CE, Quong MW, Agata Y, Murre C. E-proteins directly regulate expression of activation-induced deaminase in mature B cells. Nat Immunol. 2003;4:586–593. doi: 10.1038/ni923. [DOI] [PubMed] [Google Scholar]
- Schlissel M, Voronova A, Baltimore D. Helix-loop-helix transcription factor E47 activates germ-line immunoglobulin heavy-chain gene transcription and rearrangement in a pre-T-cell line. Genes Dev. 1991;5:1367–1376. doi: 10.1101/gad.5.8.1367. [DOI] [PubMed] [Google Scholar]
- Shi Y, Yamazaki T, Okubo Y, Uehara Y, Sugane K, Agematsu K. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. J Immunol. 2005;175:3262–3267. doi: 10.4049/jimmunol.175.5.3262. [DOI] [PubMed] [Google Scholar]
- Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nature reviews. 2009;9:185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- Sigvardsson M, O’Riordan M, Grosschedl R. EBF and E47 collaborate to induce expression of the endogenous immunoglobulin surrogate light chain genes. Immunity. 1997;7:25–36. doi: 10.1016/s1074-7613(00)80507-5. [DOI] [PubMed] [Google Scholar]
- Steger MM, Maczek C, Berger P, Grubeck-Loebenstein B. Vaccination against tetanus in the elderly: do recommended vaccination strategies give sufficient protection. Lancet. 1996;348:762. doi: 10.1016/S0140-6736(05)65680-2. [DOI] [PubMed] [Google Scholar]
- Stoecklin G, Anderson P. Posttranscriptional mechanisms regulating the inflammatory response. Advances in immunology. 2006;89:1–37. doi: 10.1016/S0065-2776(05)89001-7. [DOI] [PubMed] [Google Scholar]
- Tangye SG, Good KL. Human IgM+CD27+ B cells: memory B cells or “memory” B cells? J Immunol. 2007;179:13–19. doi: 10.4049/jimmunol.179.1.13. [DOI] [PubMed] [Google Scholar]
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–169. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- Wood JM, Schild GC, Newman RW, Seagroatt V. Application of an improved single-radial-immunodiffusion technique for the assay of haemagglutinin antigen content of whole virus and subunit influenza vaccines. Dev Biol Stand. 1977;39:193–200. [PubMed] [Google Scholar]