Abstract
Bladder reflexes evoked by stimulation of pudendal afferent nerves (PudA-to-Bladder reflex) were studied in normal and chronic spinal cord injured (SCI) adult cats to examine the reflex plasticity. Physiological activation of pudendal afferent nerves by tactile stimulation of the perigenital skin elicits an inhibitory PudA-to-Bladder reflex in normal cats, but activates an excitatory reflex in chronic SCI cats. However, in both normal and chronic SCI cats electrical stimulation applied to the perigenital skin or directly to the pudendal nerve induces either inhibitory or excitatory PudA-to-Bladder reflexes depending on stimulation frequency. An inhibitory response occurs at 3–10 Hz stimulation, but becomes excitatory at 20–30 Hz. The inhibitory reflex activated by electrical stimulation significantly (P<0.05) increases the bladder capacity to about 180% of control capacity in normal and chronic SCI cats. The excitatory reflex significantly (P<0.05) reduces bladder capacity to about 40% of control capacity in chronic SCI cats, but does not change bladder capacity in normal cats. Electrical stimulation of pudendal afferent nerves during slow bladder filling elicits a large amplitude bladder contraction comparable to the contraction induced by distension alone. A bladder volume about 60% of bladder capacity was required to elicit this excitatory reflex in normal cats; however, in chronic SCI cats a volume less than 20% of bladder capacity was sufficient to unmask an excitatory response. This study revealed the co-existence of both inhibitory and excitatory PudA-to-Bladder reflex pathways in cats before and after chronic SCI. However our data combined with published electrophysiological data strongly indicates that the spinal circuitry for both the excitatory and inhibitory PudA-to-Bladder reflexes undergoes a marked reorganization after SCI.
Keywords: urinary bladder, spinal cord injury, plasticity, cat, electrical stimulation
INTRODUCTION
In humans and animals the functions of the lower urinary tract to store and eliminate urine are controlled by neural circuits in the brain and the spinal cord (Barrington, 1921; de Groat and Ryall, 1969; de Groat, 1975; de Groat et al., 1982, 1993; Fowler et al., 2008; Kuru, 1965) that can undergo marked changes during postnatal development (Araki and de Groat, 1997; de Groat et al., 1975; de Groat, 2002; Studeny et al., 2005; Thor et al., 1989) or after SCI (de Groat et al., 1990, 1993; Kruse and de Groat, 1994; Vizzard, 2006). In neonatal kittens and rats, micturition is mediated by a spinal reflex pathway activated by afferent axons in the pudendal nerve when the mother animal licks the perigenital region of the neonate (de Groat et al., 1975; Kruse and de Groat, 1993, 1994). Isolating kittens younger than 3 weeks of age from their mother results in complete urinary retention (Thor et al., 1989). This spinal perigenital-to-bladder micturition reflex gradually disappears during postnatal development (de Groat et al., 1975) and is replaced in adult animals by a bladder-to-bladder micturition reflex mediated by a supraspinal pathway involving the pontine micturition center (PMC) (Barrington, 1921; de Groat, 1975; Fowler et al., 2008; Tai et al., 2009). In addition, in adult animals tactile stimulation of the perigenital region suppresses the supraspinal micturition reflex (de Groat, 1975; Thor et al., 1986, 1990).
SCI in neonatal animals prevents the down-regulation of the excitatory perigenital-to-bladder reflex and the emergence of the supraspinal micturition reflex during postnatal development (de Groat, 1975; Kruse and de Groat, 1993, 1994). Chronic SCI in adult animals also causes the reemergence of the excitatory perigenital-to-bladder spinal reflex (de Groat et al., 1993; Thor et al., 1986; Vizzard, 2006; Tai et al., 2006a, 2008). Similar plasticity in somato-bladder reflexes occurs in humans after chronic SCI. Genital or anal stimulation inhibits bladder activity in normal humans (Bors and Comarr, 1971; Kock and Pompeius, 1963, 1964); whereas tactile stimulation of the perianal or perigenital regions in SCI subjects enhances bladder activity and can induce micturition (Denny-Brown and Robertson, 1933; Langworthy, 1937; Rossier and Bors, 1964).
Based on previous observations using tactile perigenital stimulation, the plasticity underlying the PudA-to-Bladder reflex occurring during postnatal development or after chronic SCI could involve at least two mechanisms (de Groat et al., 1993, 1998; Vizzard, 2006). One possibility is that during postnatal development tonic descending inhibitory influences from the brain turn off the primitive excitatory reflex circuitry in the spinal cord (Araki and de Groat, 1997). Interruption of these descending connections in older animals then causes the reemergence of the neonatal reflex pattern (de Groat and Ryall, 1969, Thor et al., 1986). A second possibility is that during postnatal development, elimination of primary afferent terminations in the spinal cord (synaptic regression) (Thor et al., 1986) or pruning of excitatory interneuronal inputs to bladder parasympathetic preganglionic neurons (Araki and de Groat, 1997; de Groat et al., 1998) is responsible for postnatal loss of reflexes, whereas following SCI, restoration of synaptic connections (axonal sprouting) leads to recovery of neonatal reflexes
However, our recent studies (Tai et al., 2006b, 2007a, 2007b, 2008; Wang et al., 2008) have shown that electrical stimulation of afferent axons in the pudendal nerve in adult chronic SCI cats could either excite or inhibit the bladder depending on the frequency of stimulation. In addition, bladder excitation (Boggs et al., 2005) or excitation of bladder preganglionic neurons (de Groat and Ryall, 1969) was also reported in normal adult cats when the pudendal nerve was electrically stimulated. These experiments indicate that electrically evoked inhibitory and excitatory PudA-to-Bladder reflexes coexist in both normal and chronic SCI adult cats, which is clearly different from the results of previous experiments (de Groat, 2002) using tactile perigenital stimulation to activate pudendal afferents.
In the present study bladder reflexes induced by both tactile and electrical perigenital stimulation were elicited in adult cats with intact spinal cord or after chronic spinal cord transection to characterize SCI-induced plasticity of the PudA-to-Bladder reflexes. In addition pudendal afferent nerves were activated by electrical stimulation over a range of frequencies so that frequency response curves could be compared in normal and SCI animals. Analysis of the plasticity of the PudA-to-Bladder reflexes following chronic SCI could provide insights into the mechanisms underlying neurogenic lower urinary tract dysfunction.
METHODS
All protocols involving the use of animals in this study were approved by the Animal Care and Use Committee at the University of Pittsburgh.
Spinal cord transection and chronic care of animals
Seven female adult cats (2.8 – 3.4 kg) were spinalized under isoflurane anesthesia (2–3% in O2) using aseptic surgical techniques. After performing a dorsal laminectomy at T9–T10 vertebral level, a local anesthetic (lidocaine 1%) was applied to the surface of the spinal cord and then injected into the cord through the dura. The spinal cord was then cut completely and a piece of gel foam was placed between the cut ends (usually a separation of 2–3 mm). The muscle and skin were sutured and after full recovery from anesthesia the animal was returned to its cage. Following spinal transection, the bladder was emptied daily by manual expression. If manual expression was not successful, a sterile catheter (3.5 F) was inserted through the urethra to empty the bladder. Ketoprofen (2 mg/kg s.c., twice a day for 3 days) and antibiotics (Clavamox, 15–20 mg/kg s.c. for 7 days) were administered following surgery.
Experimental procedures in awake SCI animals
Experiments were conducted in awake animals beginning at least 4–5 weeks following spinal cord transection. The spinal cats were used for multiple experiments at a maximal frequency of twice per week. A sterile double lumen balloon catheter (7 F) was inserted through the urethra into the bladder of the chronic SCI cats without anesthesia. The balloon was distended by 2 ml of air and then positioned at the bladder neck by gently pulling the catheter back. The balloon prevented leakage of the fluid from the bladder. One lumen of the catheter was connected to a pump to infuse the bladder with sterile saline at a rate of 2–5 ml/min, and the other lumen was connected to a pressure transducer to measure the pressure change in the bladder. A pair of sterilized hook electrodes (made from 23G needles) was attached to the skin (about 1 mm penetration into the skin) on the left and right sides of the vagina approximately 1–1.5 cm from the vaginal opening. The electrodes were connected to a stimulator (S88, Grass Medical Instruments, Quincy, Massachusetts) to deliver electrical stimulation. Due to the complete spinal cord transection, the animals did not sense either bladder catheterization or electrical stimulation. During the experiment (usually 4–5 hours) the animals rested comfortably in a padded animal transport carrier. Since the animal was free to move in the carrier, bladder pressure recordings were occasionally disrupted by the animal’s movements; these recordings were discarded. At the end of the experiment the urethral catheter was withdrawn and the electrodes were detached. After each experiment the animal was given 150 mg/kg of ampicillin subcutaneously. Bladder infection rarely occurred.
Experimental procedures in anesthetized animals
A total 16 female cats (2.5 to 3.9 kg) with an intact spinal cord and the 7 female chronic SCI cats were anesthetized initially with isoflurane (2–3% in O2) for surgery and then maintained with α-chloralose (60–70 mg/kg i.v., supplemented as needed) during the recording. Heart rate and blood oxygen level were measured by a pulse oximeter (9847V, NONIN Medical, Inc., Playmouth, MN) with the sensor attached to the tongue. Systemic blood pressure was measured via a catheter in the carotid artery. Anesthetics and fluid were administrated via the ulnar vein, and airway access was secured with a tracheostomy tube. The ureters were exposed by an abdominal incision, cut, and drained externally. A double lumen catheter (5 F) was inserted through the urethra into the bladder and secured by a ligature around the urethra. One lumen of the catheter was attached to a pump to infuse the bladder with saline, and the other lumen was connected to a pressure transducer to monitor bladder activity. The bladder was infused at the rate of 2–5 ml/min. The pudendal nerve (usually on the left side) was accessed posteriorly in the sciatic notch near the base of the tail. A tripolar cuff electrode (NC223pt, MicroProbe, Inc, Gaithersburg, MD) was applied around the pudendal nerve proximal to the origin of the cutaneous branch to the perigenital region and then the muscle and skin were closed by sutures. In some of the experiments, hook electrodes as described above in the awake experiments were attached to the perigenital skin area for stimulation. The temperature of the animal was maintained at 35–37°C using a heating pad during the experiments.
Stimulation protocols
In both normal and chronic SCI cats uniphasic pulses (0.1–0.2 ms pulse width) of various frequencies (0.5–40 Hz) were delivered to the electrodes on the pudendal nerve (0.7–10 V) or the perigenital skin area (10–30 V) from a stimulator (S88) with a stimulus isolator (SIU5, Grass Medical Instruments, Quincy, Massachusetts).
In the first group of experiments, the bladder was infused with saline to one of two different volumes: (1) a volume slightly above the micturition threshold to induce large amplitude (greater than 30 cmH2O), rhythmic reflex bladder contractions (see left column in Fig. 1); or (2) a volume slightly below the micturition threshold so that large amplitude, reflex bladder contractions did not occur (see right column in Fig. 1). During the large amplitude isovolumetric bladder contractions, mechanical perigenital stimulation (MPGS) was applied by lightly stroking the perigenital skin area repeatedly (2–3 times/second for 30–180 seconds, stroke length 2–3 cm) using a cotton swab. Electrical stimulation at different frequencies was also applied to the perigenital skin area or to the pudendal nerve in order to determine the effective stimulation parameters to inhibit the isovolumetric bladder contractions. The stimulation duration was longer than the period of at least two bladder contractions in order to clearly demonstrate an inhibitory effect. The effective stimulation parameters to induce bladder contractions were determined when bladder volume was below micturition threshold and the bladder was quiescent. A stimulation duration of 20–60 seconds was used to induce bladder contractions. Different stimulation parameters were tested in a random order, but are shown in ascending intensity and/or frequency for clarity.
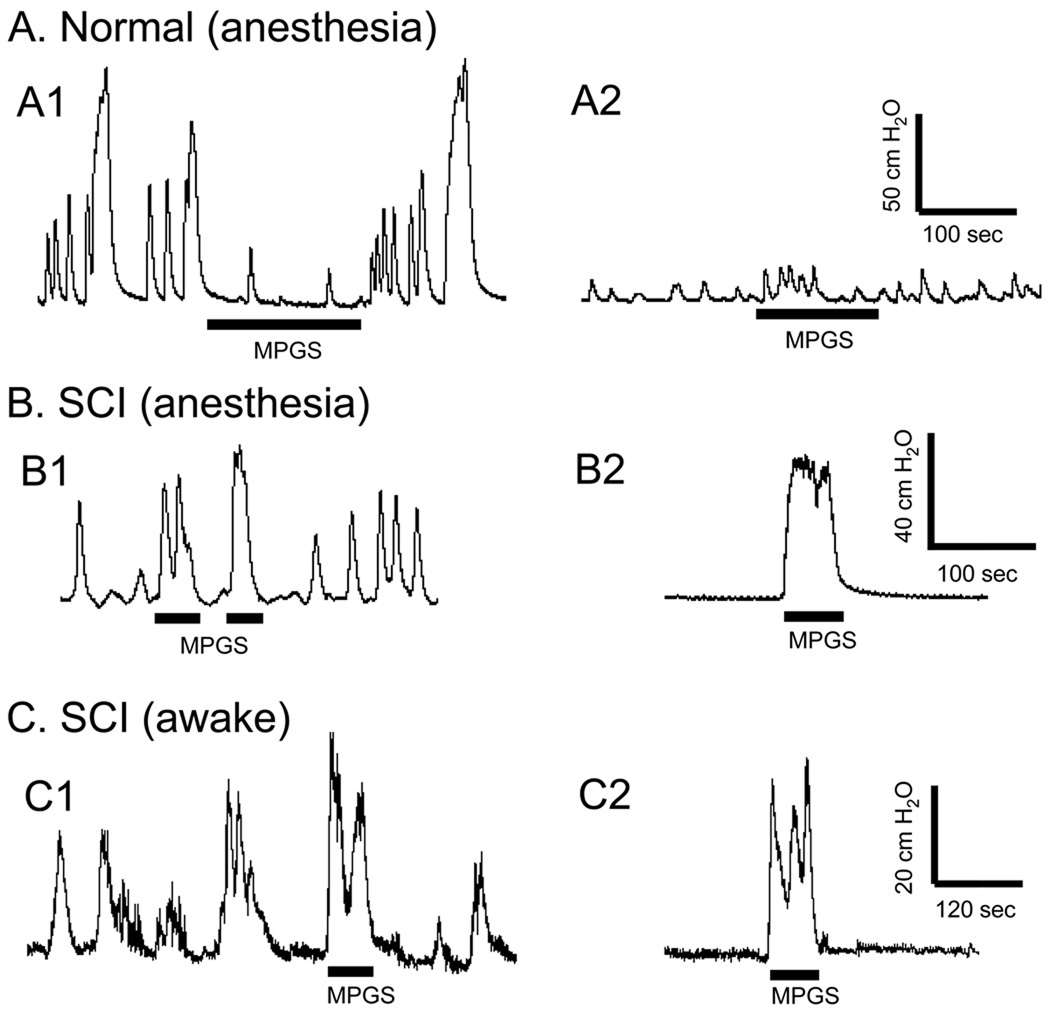
Fig. 1.
Plasticity of the perigenital-to-bladder reflex induced by chronic SCI. In normal cats (N=6) under anesthesia mechanical perigenital stimulation (MPGS) inhibited isovolumetric bladder contractions at a bladder volume exceeding the threshold volume for inducing the micturition reflex (A1). MPGS did not induce large amplitude bladder contractions when bladder was quiescent at a volume below the micturition threshold (about 90–100%) (A2). In chronic SCI cats (N=4) under anesthesia MPGS facilitated isovolumetric bladder contractions (B1). It also induced a large bladder contraction when bladder was quiescent at a low bladder volume (B2). In awake chronic SCI cats (N=4) MPGS also facilitated isovolumetric bladder contractions (C1), and induced a large bladder contraction at a low bladder volume (C2). The black bars under pressure traces indicate the stimulation duration. MPGS was performed by repeated light stroking (2–3 times/sec for 30–180 sec, stroke length 2–3 cm) of the perigenital skin area using a cotton swab.
In the second group of experiments, the most effective stimulation frequencies identified during isovolumetric recordings (3–10 Hz for inhibition, 20–30 Hz for excitation) were tested during a cystometrogram (CMG) consisting of a slow infusion of saline starting with an empty bladder until the occurrence of the first micturition contraction, which was defined as the first large amplitude (greater than 30 cmH2O), long duration (greater than 20 seconds) reflex bladder contraction. In awake chronic SCI cats the first micturition contraction was always accompanied by hindlimb stepping movements (Tai et al., 2006a, 2008; Thor et al., 1983; Wang et al., 2008). Therefore, the hindlimb stepping was also used as additional criterion to determine the occurrence of a micturition reflex contraction in awake chronic SCI cats. Bladder capacity is defined as the volume threshold for inducing the first micturition contraction during a CMG. Initially two or three control CMGs were performed without stimulation to obtain the control bladder capacity and evaluate reproducibility. Then, electrical stimulation at different frequencies was applied to the perigenital skin area or to the pudendal nerve during CMGs. The stimulation intensity determined to be effective in inhibiting isovolumetric bladder contractions was used. Inhibitory or excitatory effects were evaluated by measuring the change in bladder capacity. A control CMG was performed at the end of the test to confirm the recovery of the micturition reflex. The bladder was emptied after each CMG and a 5–10 minute rest period was inserted between CMGs to allow the bladder reflexes to recover.
In the third group of experiments, the ability of the excitatory stimulation (20–30 Hz) to induce bladder contractions at different bladder volumes was evaluated in anesthetized spinal intact cats or awake chronic SCI cats using pudendal nerve stimulation or by applying electrical stimulation to the perigential skin area. Short periods (20–50 seconds) of stimulation were applied during the CMGs as the bladder volume was increased in increments representing 5–20% of bladder capacity.
Data analysis
For the analysis of isovolumetric bladder contractions, the area under bladder pressure curve was measured during the electrical stimulation and was normalized to the measurement during the same time period prior to the stimulation (Tai et al., 2008). For the bladder contractions induced by electrical stimulation at a bladder volume below the volume for initiating a micturition reflex, the areas under the evoked bladder pressure curves were measured for different stimulation frequencies and were normalized to the maximal bladder response induced during each experimental trial. The bladder capacity was measured during each CMG and normalized to the measurement during the first control CMG. The amplitudes of bladder contractions induced by electrical stimulation during a CMG were compared at different bladder volumes that were normalized to the bladder capacity in each experiment, and then grouped into bins representing 20% increments in bladder volume. Repeated measurements in the same animal under the same conditions were averaged. The normalized data from different animals are presented as mean ± SEM. ANOVA and student t-test was used to detect statistical significance (P<0.05).
RESULTS
1. Effect of tactile perigenital stimulation on isovolumetric bladder contractions in spinal intact and chronic spinal cord injured cats
In six out of the seven normal cats under anesthesia mechanical perigenital stimulation (MPGS), which was applied by lightly stroking the perigenital skin repeatedly (2–3 times/second for 30–180 seconds, stroke length 2–3 cm) using a cotton swab, inhibited isovolumetric bladder contractions evoked by filling the bladder to a volume slightly above the micturition threshold volume (Fig. 1A1). MPGS did not induce large amplitude (greater than 30 cmH2O), long duration (greater than 20 seconds) reflex bladder contraction when bladder volume was low and bladder was quiescent (Fig. 1A2), except in one cat where it induced a large (amplitude 30–40 cmH2O) bladder contraction. However in chronic SCI cats (N = 4) under anesthesia or in awake conditions MPGS evoked a large bladder contraction (40–60 cmH2O) when the bladder volume was large and during on-going reflex bladder contractions (Figs. 1B1 and 1C1) or when the bladder volume was small and the bladder was quiescent (Figs. 1B2 and 1C2).
2. Effect of electrical stimulation of the perigenital region or the pudendal nerve at different frequencies in normal and chronic SCI cats
In anesthetized normal cats the frequency dependence of the inhibitory PudA-to-Bladder reflex was examined by electrical stimulation at frequencies between 0.5–40 Hz (Fig. 2–3). Electrical perigenital stimulation (EPGS, 10–30 V, 0.2 ms) at frequencies of 3–7 Hz significantly (P<0.05) suppressed isovolumetric bladder contractions (Fig. 2A and Fig. 3), while electrical pudendal nerve stimulation (EPNS, 1–6 V, 0.1 ms) at frequencies of 5–10 Hz significantly (P<0.05) inhibited isovolumetric bladder contractions (Figs. 2B and Fig.3). Stimulation frequencies below 1 Hz or above 20 Hz were not effective in inhibiting isovolumetric bladder contractions (Figs. 2–3). However, EPNS (1–10 V, 0.1 ms) or EPGS (30 V, 0.2 ms) at frequencies of 20 Hz and 30 Hz induced an excitatory reflex when bladder volume was below the micturition threshold volume (about 90–100%) and the bladder was quiescent, while at other frequencies (0.5–10 Hz and 40 Hz) it was ineffective (Fig. 4).
Fig. 2.
The inhibitory PudA-to-Bladder reflex is dependent on stimulation frequency in normal cats under anesthesia. A. Electrical perigenital stimulation (EPGS: 20 V, 0.2 ms) at frequencies of 1–10 Hz completely inhibited the isovolumetric bladder contractions. B. Electrical pudendal nerve stimulation (EPNS: 1 V, 0.1 ms) at 3–10 Hz completely inhibited the bladder activity. The black bars under pressure traces indicate the stimulation duration.
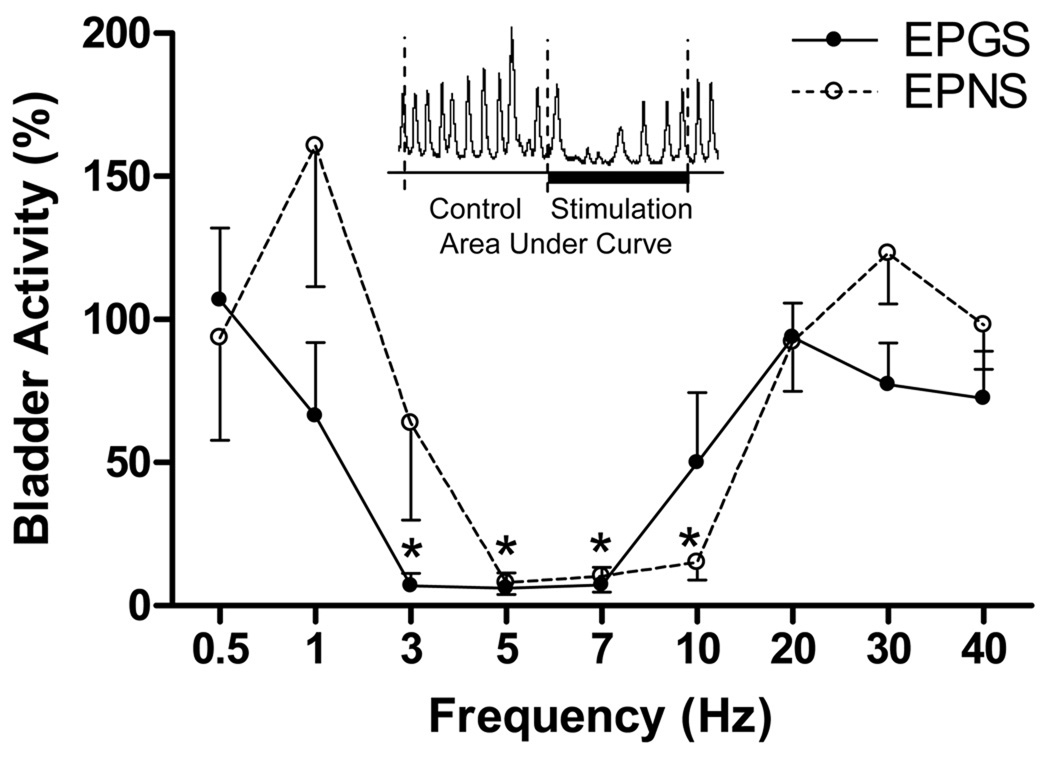
Fig. 3.
Frequency range effective in inhibiting isovolumetric bladder contractions in normal cats under anesthesia. The bladder activity (%) indicates the area under curve of the isovolumetric bladder contractions during stimulation that was normalized to the measurement during the same time length prior to the stimulation (see inserted figure). Electrical perigenital stimulation (EPGS: 10–30 V, 0.2 ms, N=6) at frequencies of 3–7 Hz and electrical pudendal nerve stimulation (EPNS: 1–6 V, 0.1 ms, N=8) at frequencies of 5–10 Hz significantly suppressed isovolumetric bladder contractions. * indicates statistically significant difference from 100%.
Fig. 4.
The excitatory PudA-to-Bladder reflex is dependent on stimulation frequency in normal cats under anesthesia at a bladder volume about 90–100% of the micturition threshold volume. A. Short duration (60 sec) electrical pudendal nerve stimulation (EPNS: 1 V, 0.1 ms) induced large amplitude bladder contractions at frequencies of 20–30 Hz. B. Similar results were obtained by electrical perigenital stimulation (EPGS: 30 V, 0.2 ms). B. Summarized results indicate that EPNS (1–10 V, 0.1 ms, N=10) or EPGS (30 V, 0.2 ms, N=3) at 20–30 Hz induced significantly larger bladder contractions than other stimulation frequencies. The black bars under pressure traces indicate the stimulation duration. * indicates statistically significant difference (P<0.05).
In chronic SCI cats as reported in our previous publications (Tai et al. 2006b, 2007a, 2007b, 2008; Wang et al. 2008), the EPNS and EPGS evoked bladder reflexes were inhibitory at stimulation frequencies of 3–10 Hz, but excitatory at 20–30 Hz.
3. Volume dependence of the excitatory PudA-to-Bladder reflex
Bladder volume influenced the excitatory reflex differently in normal and chronic SCI cats (Fig. 5). In anesthetized normal cats when the bladder volume was greater than 60% of the bladder capacity short duration (20–40 sec) EPNS (1–6 V, 0.1 ms, 20–30 Hz) or EPGS (30 V, 0.2 ms, 20 Hz) induced large amplitude bladder contractions, which were not significantly (P>0.05) different from the amplitude of micturition contractions (40–100 cmH2O) induced by bladder distension alone (Figs. 5A, 5B, and 5D). At smaller bladder volumes (less than 60% of the capacity) EPNS or EPGS induced bladder contractions of significantly (P<0.05) smaller amplitude (10–40 cmH2O) when compared to the contractions induced by bladder distention at 100–110% of bladder capacity (Fig. 5D) in the same group of animals. However, in awake chronic SCI cats activation of the excitatory reflex by short duration (20–40 sec) EPGS (11–30 V, 0.2 ms, 30 Hz) induced large bladder contractions (30–60 cmH2O) even at a bladder volume less than 20% of the bladder capacity (Figs. 5C and 5D), indicating that the excitatory PudA-to-Bladder reflex is less dependent on bladder volume in chronic SCI cats.
Fig. 5.
Volume dependent response of the excitatory PudA-to-Bladder reflex during CMG. In a normal cat (A) under anesthesia short duration (30 sec) electrical pudendal nerve stimulation (EPNS: 20 Hz, 6 V, 0.1 ms) induced bladder contractions gradually increasing in amplitude as the bladder volume increased. Similar results was also obtained in another normal cat under anesthesia (B) by short duration (30 sec) electrical perigenital stimulation (EPGS: 20 Hz, 30V, 0.2 ms). However, in a chronic SCI cat (C) under awake condition short duration (20 sec) electrical perigenital stimulation (EPGS: 30 Hz, 8V, 0.2 ms) induced large bladder contractions at a bladder volume less than 20% of the bladder capacity. The thick black bars under pressure traces indicate the stimulation duration. The arrows indicate the start and stop of bladder infusion. Summarized results (D) indicate that bladder response to short duration (20–40 sec) EPNS (20–30 Hz, 1–6 V, 0.1 ms, N=8) or EPGS (20 Hz, 30 V, 0.2 ms, N=3) is dependent on bladder volume in normal cats. However, it is volume independent in awake chronic SCI cats when short duration (20–40 sec) EPGS (30 Hz, 11–30 V, 0.2 ms, N=3) is applied. * indicate a statistically significant (P<0.05) difference from the reflex bladder contraction amplitude induced by bladder distension (i.e. at 100–110% of bladder capacity).
4. Micturition threshold volume is modulated by EPNS
EPNS was also tested during CMGs to evaluate the effects of different frequencies of nerve stimulation on the bladder volume threshold for inducing a micturition reflex (i.e., bladder capacity). Low frequency EPNS (3–10 Hz, Fig.3) applied during bladder filling increased the bladder capacity in both normal and chronic SCI cats (Fig. 6). In anesthetized normal cats EPNS (1–2 V, 0.1 ms, 3–10 Hz) significantly (P<0.05) increased the bladder capacity to 177.4±28.4% of the control capacity (Figs. 6A and 6C). In anesthetized chronic SCI cats EPNS (0.7–5 V, 0.1 ms, 3–7 Hz) elicited a similar increase in bladder capacity (to 176.6±7.1%; P<0.05) (Figs. 6B and 6C).
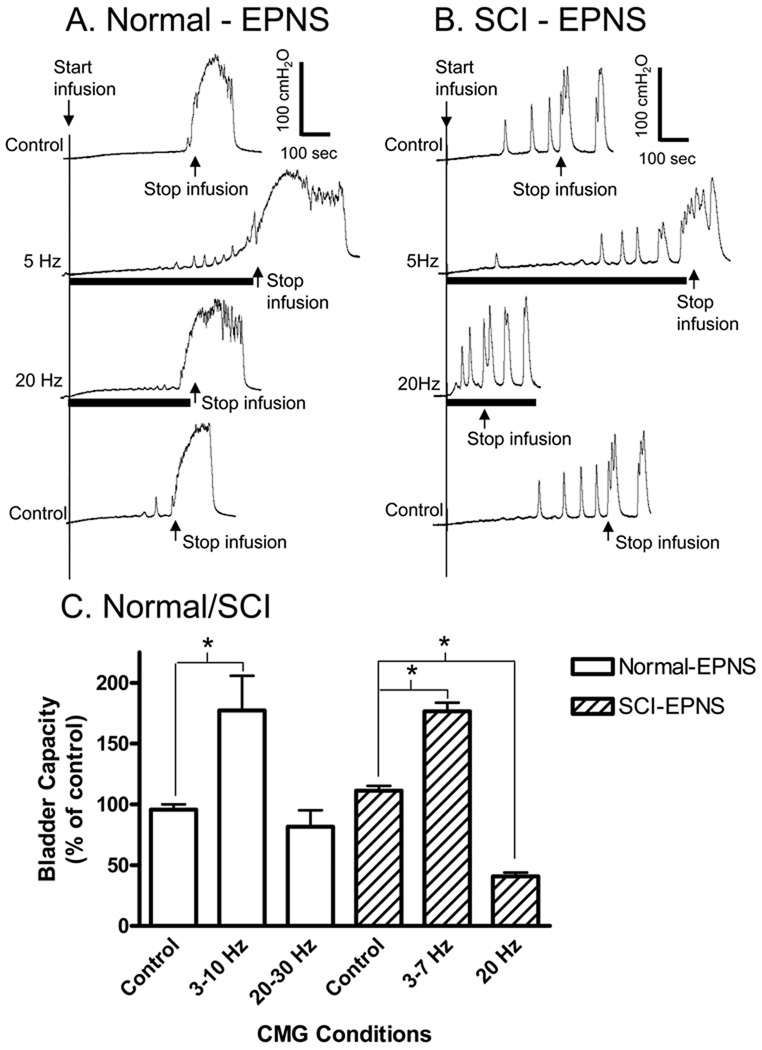
Fig. 6.
Influence of the inhibitory or excitatory somato-bladder reflexes on bladder capacity in normal and chronic SCI cats under anesthesia. In a normal cat (A) 5 Hz electrical pudendal nerve stimulation (EPNS: 1 V, 0.1 ms) applied during CMG increased bladder capacity, but 20 Hz stimulation was ineffective. However, in a chronic SCI cat (B) 20 Hz EPNS (0.7 V, 0.1 ms) decreased bladder capacity in addition to increasing the capacity during 5 Hz stimulation. Summarized results (C) from 10 normal and 3 chronic SCI cats indicate a difference of the excitatory reflex in normal and chronic SCI cats. The black bars under pressure traces indicate the stimulation duration. Arrows indicate the start and stop of the bladder infusion (A: 1ml/min, B: 5 ml/min). * indicates statistically significant difference (P<0.05).
On the other hand, EPNS at 20–30 Hz, which elicited bladder contractions under constant bladder volume conditions (Fig. 4), had different effects on bladder capacity in normal and chronic SCI cats (Fig. 6). In anesthetized chronic SCI cats EPNS (0.7–5 V, 0.1 ms, 20 Hz) significantly (P<0.05) reduced the bladder capacity to 40.7±3.4% of the control capacity (Figs. 6B and 6C). However, in anesthetized normal cats EPNS (1–2 V, 0.1 ms, 20–30 Hz) did not significantly change bladder capacity (Figs. 6A and 6C).
DISCUSSION
This study which examined plasticity of PudA-to-Bladder reflexes after SCI revealed that electrical stimulation of pudendal nerve afferent axons (EPNS or EPGS) at different frequencies can selectively activate inhibitory or excitatory PudA-to-Bladder reflexes in normal (Fig. 2–4) as well as chronic SCI cats (Tai et al., 2006b, 2007a, 2007b, 2008; Wang et al., 2008). However, activation of a subpopulation of pudendal nerve afferents by tactile stimulation of the perigenital skin area (MPGS) only induced an inhibitory reflex in normal cats and an excitatory reflex in chronic SCI cats (Fig. 1). Thus the neuroplasticity that occurs in MPGS-evoked bladder reflexes after SCI, which has been used as a model to study rewiring of spinal cord circuitry in paraplegic animals (Araki and de Groat, 1997; de Groat and Ryall, 1969; de Groat et al., 1981), is less prominent when a more diverse population of pudendal nerve afferent axons is activated by EPNS or EPGS.
Some responses to EPNS were not changed after SCI. For example, EPNS elicited inhibition and excitation in normal as well as chronic SCI cats and the range of EPNS frequencies for inducing inhibition (3–10 Hz) or excitation (20–30 Hz) were similar in both preparations. Furthermore low frequency EPNS increased bladder capacity to the same extent in both normal and chronic SCI cats (Fig.6).
However some differences were also noted when comparing the effects of EPNS in normal and chronic SCI animals. High frequency EPNS reduced bladder capacity in chronic SCI cats but not in normal cats (Fig. 6). In addition the EPNS/EPGS-evoked excitatory reflexes exhibited a different sensitivity to the state of the bladder in normal and chronic SCI cats (Fig. 5). A bladder volume equal to 60% of bladder capacity was required to unmask the EPNS/EPGS-evoked bladder contraction in normal cats, but less than 20% of bladder capacity was sufficient in chronic SCI cats (Fig. 5). These data indicate that SCI-induced plasticity of PudA-to-Bladder reflexes is prominent in central pathways activated by tactile stimulation of a subpopulation of cutaneous afferent nerves but is more subtle or does not occur in other pathways activated by electrical stimulation of pudendal afferent nerves innervating deeper tissues such as the urethra, anal canal and sphincter muscles.
After chronic SCI the bladder inhibitory response to MPGS was eliminated (Fig. 1) but the EPNS- or EPGS-evoked inhibitory responses persisted (Fig. 6). This suggests that pudendal afferents elicit bladder inhibition by at least two different mechanisms: one that targets spinal pathways (Fig. 7B) and another that targets the supraspinal micturition reflex pathway (Fig. 7A). Inhibition at the spinal level must occur in part by direct inhibitory input to the bladder parasympathetic preganglionic neurons (PPGN) (Fig. 7) because previous electrophysiological studies (de Groat and Ryall, 1969) revealed that EPNS elicits short latency inhibitory postsynaptic potentials (IPSPs) in PPGN and inhibits the firing of PPGN induced by iontophoretic application of an excitatory amino acid as well as reflex firing induced by bladder distension (de Groat et al., 1982). On the other hand, MPGS inhibits reflex firing but does not inhibit amino acid evoked firing (de Groat, 1971) indicating that MPGS suppresses micturition by inhibiting transmission at interneuronal synapses on the reflex pathway prior to the PPGN, eg., at the level of spinal tract neurons on the ascending limb of the spinobulbospinal (SBS) pathway (see Fig. 7A). Inhibition at this site or in the brain stem could be responsible for an inhibition of PPGN reflex firing that occurs at long latencies after EPNS (de Groat et al., 1982). This inhibition does not occur at the level of the PPGN because it does alter amino acid induced PPGN firing (de Groat et al., 1982).
Fig. 7.
The possible neural pathways involved in PudA-to-Bladder reflexes before (A) and after (B) spinal cord injury (SCI). To simplify the diagrams, dashed lines on the left side of each figure are used to indicate projections of different pudendal afferent pathways to common spinal targets.
However activation of other pudendal nerve afferent pathways by mechanical stimulation of the ano-rectal mucosa inhibits both reflex and amino acid evoked firing of bladder PPGN and therefore mimics the effect of EPNS. In addition stimulation of ano-rectal afferents inhibits bladder PPGN reflex firing in both normal and chronic SCI cats, whereas MPGS only inhibits firing and bladder activity in normal cats (de Groat, 1971). These results indicate that activation of cutaneous perigenital afferents inhibits the SBS reflex pathway that regulates micturition in normal animals, while activation of pudendal afferent nerves innervating other organs (eg., sphincter muscles and bowel) inhibits spinal as well as SBS reflexes by directly inhibiting the bladder PPGN as well as interneuronal synapses on the SBS micturition reflex pathway in the spinal cord (Fig. 7) or in the brain.
Although somato-bladder inhibition can occur by multiple mechanisms it is noteworthy that inhibition induced by EPGS or EPNS occurred at the same low frequencies (Fig. 2; 3–10 Hz) in both normal and chronic SCI cats suggesting that the inhibitory pathway is organized in the spinal cord (Fig. 7). The loss of inhibition at higher stimulation frequencies (Fig. 3) is consistent with data obtained in electrophysiological studies of bladder PPGN in the cat showing that EPNS-evoked IPSPs were prominent at 5 Hz stimulation but abolished during higher frequency stimulation (20 Hz) (de Groat and Ryall, 1969). The frequency response characteristics of EPNS inhibition may be related to the inability of synaptic transmission in the inhibitory pathway to follow high frequencies or that stimulation above 10 Hz activates parallel circuitry that turns off the inhibitory pathway (i.e., dysinhibition). Because the EPNS-evoked IPSPs occur at short latency (5 msec) this type of somato-bladder inhibition probably occurs via a spinal disynaptic pathway consisting of excitatory synaptic inputs from pudendal afferent axons to inhibitory interneurons which in turn synapse with PPGN (Fig. 7).
Although the bladder excitatory effects elicited by pudendal afferents were induced by similar high frequencies of stimulation (HF, 20–40 Hz) in both normal (Fig. 4) and chronic SCI cats (Tai et al., 2006b, 2008) two observations indicate that the excitation occurs by at least two different mechanisms (see pathways labeled HF in Fig. 7). First, a significantly larger bladder volume was necessary for unmasking the EPNS evoked bladder contraction in normal cats (> 60% of the micturition volume threshold) than in chronic SCI cats (20% of the micturition volume threshold). Second, high frequency EPNS significantly decreased bladder capacity in chronic SCI cats but was ineffective in normal cats suggesting that the gating circuit for initiating micturition was only affected in cats with a transected spinal cord.
The bladder excitatory response elicited in the present experiments in chronic SCI cats by EPNS is probably mediated by a spinal pathway identified in previous electrophysiological studies in SCI cats (de Groat and Ryall, 1969; de Groat et al., 1981). In those experiments EPNS elicited a short latency (5 msec) excitatory postsynaptic potential (EPSP) in bladder PPGN and a reflex discharge on bladder postganglionic nerves that occurred with a central delay that was considerably shorter (15–30 msec) than the central delay of the reflex in normal cats (60–75 msec). The early reflex in chronic SCI cats occurred only at high frequencies of stimulation (10–20 Hz) similar to those required to induce bladder contractions in the present experiments and was never detected in normal animals. However similar excitatory reflexes have been detected in neonatal kittens with intact spinal cords and after acute or chronic SCI (de Groat et al., 1981). Thus it has been proposed that the short latency PudA-to-Bladder reflex mediates voiding in neonatal kittens in response to MPGS induced by the mother licking the perineum. Because bladder-to-bladder reflexes are absent during the early postnatal period in various animals (cats, dogs, rats) the excitatory spinal perigenital-to-bladder reflex activated by the mother is essential for survival of the newborn. This spinal reflex is down-regulated during postnatal development as the brain assumes control of circuitry in the spinal cord (Araki and de Groat, 1997; de Groat, 2002). However after SCI the reflex can re-emerge when regulatory input from the brain is eliminated. Thus the effectiveness of the spinal EPNS-evoked excitatory reflex to reduce bladder capacity and induce bladder contractions at low bladder volumes in SCI cats is consistent with its putative function to induce voiding in neonatal animals.
The increased efficacy of EPNS to influence micturition in chronic SCI cats may also be related to the change in the bladder reflex pathway after SCI. Micturition in normal cats is triggered by Aδ bladder afferents (de Groat and Ryall, 1969), while in chronic SCI cats it is triggered by C-fiber bladder afferents (Chancellor and de Groat, 1999; Cheng et al., 1999; de Groat et al., 1981, 1990). Based on the difference in the effect of EPNS on bladder capacity in normal and SCI cats it seems likely that pudendal afferent activity facilitates the processing of C-fiber bladder afferent input but not Aδ afferent input and thereby facilitates the initiation of the micturition reflex and lowers bladder capacity only in SCI cats (Fig. 7). As shown in figure 7B convergence of pudendal afferents onto excitatory interneurons receiving inputs from bladder C-fiber afferents could underlie the facilitatory effect of EPNS on bladder capacity.
Morphological changes in pudendal primary afferent projections to the sacral spinal cord occur during postnatal development and after spinal cord injury in the cat (Thor et al., 1986) suggesting that primary afferent pruning and sprouting, respectively, contributes to the downregulation and upregulation of the EPNS-evoked excitatory bladder reflex. In addition electrophysiological studies using patch clamp recording in the neonatal rat spinal cord slice preparation indicate that synaptic pruning and sprouting at interneuronal excitatory synapses on PPGN are also involved in developmental and spinal injury induced plasticity (Araki and de Groat, 1997). Enhanced synaptic connections between excitatory interneurons (interneuron #5 in Fig. 7B) and bladder PPGN that are weak or not present in normal animals (Fig. 7A) may contribute to the emergence of both PudA-to-Bladder and C-fiber-evoked bladder reflexes (Fig. 7B). However the PudA-to-Bladder spinal excitatory pathway is not completely eliminated in adult cats, because it can be demonstrated after acute treatment with a serotonergic agonist (Thor et al., 1990) or acute transection of the spinal cord (Boggs et al., 2005). Both of these conditions may unmask the excitatory reflex by removing tonic supraspinal inhibition.
The bladder excitatory effect of EPNS in cats with an intact spinal cord must occur via a different mechanism. Previous electrophysiological studies in normal cats (de Groat and Ryall, 1969) showed that EPNS elicited long latency firing in bladder PPGN and in bladder postganglionic nerves. This firing resembled the SBS micturition reflex firing evoked by Aδ bladder afferents. The EPNS and Aδ-fiber evoked reflexes were only elicited when the bladder was distended and both were abolished by acute or chronic transection of the spinal cord at the thoracic level (de Groat and Ryall, 1969; de Groat et al., 1982). This indicates that a subpopulation of pudendal afferents in addition to bladder afferents can modulate the SBS micturition reflex pathway (Fig.7A). Barrington described a similar supraspinal somato-bladder reflex pathway with an afferent limb in the pudendal nerve that was activated by passage of fluid through the urethra (Barrington, 1931, 1941). Thus the EPNS evoked responses in normal cats could be elicited by activation of these urethral afferents in the pudendal nerve. According to Barrington the function of the urethra-to-bladder excitatory reflex is to facilitate voiding. It has never been shown that it can initiate voiding. Thus the failure of high frequency EPNS to reduce bladder capacity in normal cats is consistent with the view that it should enhance an ongoing micturition reflex but not facilitate the initiation of micturition by lowering the bladder volume threshold for triggering a reflex. The possible sites of interaction between the bladder-to-bladder reflex and the putative urethral-to-bladder reflex are the pontine micturition center (Fig. 7A) or the descending limb of the micturition reflex pathway.
It is noteworthy that tactile and electrical stimulation may have activated different population of pudendal nerve fibers in a very different way. MPGS mainly activates the mechano-sensitive pudendal afferent nerves from the skin, whereas EPGS could activate both mechano- and non-mechano- sensitive pudendal afferent nerves from both skin and muscles. EPNS can potentially activate all types of pudendal nerve fibers. Furthermore, pudendal nerves are activated asynchronously by MPGS, but they are activated synchronously by each electrical pulse during EPGS or EPNS. The frequency of pudendal afferent firing can be selected by EPGS or EPNS, but MPGS may activate pudendal afferent nerves with a large range of firing frequencies.
In summary, this study revealed that electrical stimulation of the pudendal nerve which activates afferent pathways to multiple organs elicits inhibitory and excitatory bladder reflexes that are mediated by multiple mechanisms (Fig. 7). Some of these mechanisms are unchanged by SCI and others are dramatically altered or eliminated. The inhibitory reflexes have been intensively studied in both animals (Fall et al., 1978; Lindstrom et al., 1983; Walter et al., 1993) and humans (Fall and Lindstrom, 1991; Kirkham et al., 2001; Previnaire et al., 1996, 1998; Vodusek et al., 1986, 1988; Wheeler et al., 1992), and are used clinically to suppress bladder overactivity (Peters et al., 2005, 2007). However the potential of the excitatory reflexes to improve voiding efficiency was only recently explored (Boggs et al., 2005; Shefchyk and Buss, 1998; Tai et al., 2006b, 2007a, 2007b, 2008; Wang et al., 2008). Most clinical studies of electrical stimulation of dorsal penile/clitoral nerve (a branch of pudendal nerve) used 5–15 Hz frequency for the purpose of bladder inhibition (Kirkham et al, 2001; Previnaire et al, 1996, 1998; Vodusek et al, 1986; Wheeler et al., 1992). Additional clinical studies to reveal the excitatory effect of dorsal penile/clitoral nerve stimulation at a high frequency (20–30 Hz) is needed. Further investigation of the central neural mechanisms underlying the plasticity of PudA-to-Bladder reflexes after spinal injury should provide a more detailed understanding of the pathophysiology of neurogenic lower urinary tract dysfunction and promote the development of new therapies (Kessler and Fowler, 2008).
Research Highlights
Inhibitory and excitatory pudendal-to-bladder reflexes exist before and after SCI
Electrical stimulation of pudendal afferent nerves can activate both reflexes
The inhibitory reflex is unchanged after SCI
The excitatory reflex is volume-dependent before SCI but independent after SCI
The bladder reflex evoked by tactile genital stimulation changes markedly after SCI
ACKNOWLEDGEMENTS
This study is supported by the NIH grants R01-DK-068566, R01-DK-090006, and R01-DK-077783, and by Christopher and Dana Reeve Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Araki I, de Groat WC. Developmental synaptic depression underlying reorganization of visceral reflex pathways in the spinal cord. J. Neurosci. 1997;17:8402–8407. doi: 10.1523/JNEUROSCI.17-21-08402.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington FJF. The relation of the hind-brain to micturition. Brain. 1921;44:23–53. [Google Scholar]
- Barrington FJF. The component reflexes of micturition in the cats. Parts I and II. Brain. 1931;54:177–188. [Google Scholar]
- Barrington FJF. The component reflexes of micturition in the cats. Parts III. Brain. 1931;64:239–243. [Google Scholar]
- Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Spinal micturition reflex mediated by afferents in the deep perineal nerve. J. Neurophysiol. 2005;93:2688–2697. doi: 10.1152/jn.00978.2004. [DOI] [PubMed] [Google Scholar]
- Bors E, Comarr A. Neurological Urology, Physiology of Micturition, its Neurological Disorders and Sequelae. Baltimore: University Park Press; 1971. [Google Scholar]
- Chancellor MB, de Groat WC. Intravesical capsaicin and resiniferatoxin therapy: spicing up the ways to treat the overactive bladder. J. Urol. 1999;162:3–11. doi: 10.1097/00005392-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Cheng CL, Liu JC, Chang SY, Ma CP, de Groat WC. Effect of capsaicin on the micturition reflex in normal and chronic spinal cord-injured cats. Am. J. Physiol. 1999;277:R786–R794. doi: 10.1152/ajpregu.1999.277.3.R786. [DOI] [PubMed] [Google Scholar]
- de Groat WC. Inhibition and excitation of sacral parasympathetic neurons by visceral and cutaneous stimuli in the cat. Brain Res. 1971;33:499–503. doi: 10.1016/0006-8993(71)90125-9. [DOI] [PubMed] [Google Scholar]
- de Groat WC. Nervous control of the urinary bladder of the cat. Brain Res. 1975;87:201–211. doi: 10.1016/0006-8993(75)90417-5. [DOI] [PubMed] [Google Scholar]
- de Groat WC. Plasticity of bladder reflex pathways during postnatal development. Physiol. Behav. 2002;77:689–692. doi: 10.1016/s0031-9384(02)00919-8. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Booth AM, Milne RJ, Roppolo JR. Parasympathetic preganglionic neurons in the sacral spinal cord. J. Auto. Nerv. Syst. 1982;5:23–43. doi: 10.1016/0165-1838(82)90087-x. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. The Autonomic Nervous System, Nervous Control of the Urogenital System. London, U.K: Harwood Academic Publishers; 1993. pp. 227–289. [Google Scholar]
- de Groat WC, Douglas JW, Glass J, Simonds W, Weimer B, Werner P. Changes in somato-vesical reflexes during postnatal development in the kitten. Brain Res. 1975;94:150–154. doi: 10.1016/0006-8993(75)90884-7. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Araki I, Vizzard MA, Yoshiyama M, Yoshimura N, Sugaya K, Tai C, Roppolo JR. Developmental and injury induced plasticity in the micturition reflex pathway. Behavioural Brain Res. 1998;92:127–140. doi: 10.1016/s0166-4328(97)00185-x. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, Steers W, Roppolo JR. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J. Auto. Nerv. Syst. 1990;30 suppl.:S71–S77. doi: 10.1016/0165-1838(90)90105-r. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J. Auto. Nerv. Syst. 1981;3:135–160. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Ryall RW. Reflexes to sacral parasympathetic neurones concerned with micturition in the cat. J. Physiol. 1969;200:87–108. doi: 10.1113/jphysiol.1969.sp008683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny-Brown D, Robertson EG. The state of the bladder and its sphincters in complete transverse lesions of the spinal cord and cauda epuina. Brain. 1933;56:397–462. [Google Scholar]
- Fall M, Erlandson BE, Carlsson CA, Lindstrom S. The effect of intravaginal electrical stimulation on the feline urethra and urinary bladder: neuronal mechanisms. Scand. J. Urol. Nephrol. 1978;44 suppl.:19–30. [PubMed] [Google Scholar]
- Fall M, Lindstrom S. Electrical stimulation: A physiologic approach to the treatment of urinary incontinence. Urol. Clin. North Am. 1991;18:393–407. [PubMed] [Google Scholar]
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat. Rev. Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler TM, Fowler CJ. Sacral neuromodulation for urinary retention. Nat. Clin. Pract. Urol. 2008;5:657–666. doi: 10.1038/ncpuro1251. [DOI] [PubMed] [Google Scholar]
- Kirkham APS, Shah NC, Knight SL, Shah PJR, Craggs MD. The acute effects of continuous and conditional neuromodulation on the bladder in spinal cord injury. Spinal Cord. 2001;39:420–428. doi: 10.1038/sj.sc.3101177. [DOI] [PubMed] [Google Scholar]
- Kock NG, Pompeius R. Inhibition of vesical motor activity induced by anal stimulation. Acta Chir. Scand. 1963;126:244–250. [PubMed] [Google Scholar]
- Kock NG, Pompeius R. Studies on the nature of the rhythmic activity of the human bladder. Invest. Urol. 1964;1:253–263. [PubMed] [Google Scholar]
- Kruse MN, de Groat WC. Spinal pathways mediate coordinated bladder/urethral sphincter activity during reflex micturition in decerebrate and spinalezed neonatal rats. Neurosci. Lett. 1993;152:141–144. doi: 10.1016/0304-3940(93)90503-d. [DOI] [PubMed] [Google Scholar]
- Kruse MN, de Groat WC. Consequences of spinal cord injury during the neonatal period on micturition reflexes in the rat. Exp. Neurol. 1994;125:87–92. doi: 10.1006/exnr.1994.1010. [DOI] [PubMed] [Google Scholar]
- Kuru M. Nervous control of micturition. Physiol. Rev. 1965;45:425–494. doi: 10.1152/physrev.1965.45.3.425. [DOI] [PubMed] [Google Scholar]
- Langworthy OR. A curious illustration of “mass reflex” and involuntary micturition following injury of the spinal cord. Bull. Johns Hopk. Hosp. 1937;60:204–214. [Google Scholar]
- Lindstrom S, Fall M, Carlsson CA, Erlandson BE. The neurophysiological basis of bladder inhibition in response to intravaginal electrical stimulation. J. Urol. 1983;129:405–410. doi: 10.1016/s0022-5347(17)52127-8. [DOI] [PubMed] [Google Scholar]
- Peters KM, Feber KM, Bennett RC. Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol. Urodyn. 2005;24:643–647. doi: 10.1002/nau.20174. [DOI] [PubMed] [Google Scholar]
- Peters KM, Feber KM, Bennett RC. A prospective, single-blind, randomized crossover trial of sacral vs pudendal nerve stimulation for interstitial cystitis. BJU Int. 2007;100:835–839. doi: 10.1111/j.1464-410X.2007.07082.x. [DOI] [PubMed] [Google Scholar]
- Previnaire JG, Soler JM, Perrigot M. Is there a place for pudendal nerve maximal electrical stimulation for the treatment of detrusor hyperreflexia in spinal cord injury patients? Spinal Cord. 1998;36:100–103. doi: 10.1038/sj.sc.3100440. [DOI] [PubMed] [Google Scholar]
- Previnaire JG, Soler JM, Perrigot M, Boileau G, Delahaye H, Schumacker P, Vanvelcenaher J, Vanhee JL. Short-term effect of pudendal nerve electrical stimulation on detrusor hyperreflexia in spinal cord injury patients: importance of current strength. Paraplegia. 1996;34:95–99. doi: 10.1038/sc.1996.17. [DOI] [PubMed] [Google Scholar]
- Rossier A, Bors E. Detrusor responses to perianal and rectal stimulation in patients with spinal cord injuries. Urol. Int. 1964;18:181–190. doi: 10.1159/000279237. [DOI] [PubMed] [Google Scholar]
- Studeny S, Torabi A, Vizzard MA. P2X2 and P2X3 receptor expression in postnatal and adult rat urinary bladder and lumbosacral spinal cord. Am. J. Physiol. Integr. Comp. Physiol. 2005;289:R1155–R1168. doi: 10.1152/ajpregu.00234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefchyk SJ, Buss RR. Urethral pudendal afferent-evoked bladder and sphincter reflexes in decerebrate and acute spinal cats. Neurosci. Lett. 1998;244:137–140. doi: 10.1016/s0304-3940(98)00155-4. [DOI] [PubMed] [Google Scholar]
- Tai C, Miscik CL, Ungerer TD, Roppolo JR, de Groat WC. Suppression of bladder reflex activity in chronic spinal cord injured cats by activation of serotonin 5-HT1A receptors. Exp. Neurol. 2006a;199:427–437. doi: 10.1016/j.expneurol.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Tai C, Shen B, Wang J, Chancellor MB, Roppolo JR, de Groat WC. Inhibitory and Excitatory perigenital-to-bladder spinal reflexes in the cats. Am. J. Physiol. Renal Physiol. 2008;294:F591–F602. doi: 10.1152/ajprenal.00443.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai C, Smerin SE, de Groat WC, Roppolo JR. Pudendal-to-bladder reflex in chronic spinal-cord-injured cats. Exp. Neurol. 2006b;197:225–234. doi: 10.1016/j.expneurol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Tai C, Wang J, Jin T, Wang P, Kim SG, Roppolo JR, de Groat WC. Brain switch for reflex micturition control detected by fMRI in rats. J. Neurophysiol. 2009;102:2719–2730. doi: 10.1152/jn.00700.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai C, Wang J, Wang X, de Groat WC, Roppolo JR. Bladder inhibition and voiding induced by pudendal nerve stimulation in chronic spinal cord injured cats. Neurourol. Urodyn. 2007a;26:570–577. doi: 10.1002/nau.20374. [DOI] [PubMed] [Google Scholar]
- Tai C, Wang J, Wang X, Roppolo JR, de Groat WC. Voiding reflex in chronic spinal cord injured cats induced by stimulating and blocking pudendal nerves. Neurourol. Urodyn. 2007b;26:879–886. doi: 10.1002/nau.20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor KB, Blais DP, de Groat WC. Behavioral analysis of the postnatal development of micturition in kittens. Dev. Brain Res. 1989;46:137–144. doi: 10.1016/0165-3806(89)90151-x. [DOI] [PubMed] [Google Scholar]
- Thor KB, Hisamitsu T, de Groat WC. Unmasking of a neonatal somatovesical reflex in adult cats by the serotonin autoreceptor agonist 5-methoxy-N,N-dimethyltryptamine. Dev. Brain Res. 1990;54:35–42. doi: 10.1016/0165-3806(90)90062-4. [DOI] [PubMed] [Google Scholar]
- Thor KB, Kawatani M, de Groat WC. Plasticity in the reflex pathways to the lower urinary tract of the cat during postnatal development and following spinal cord injury. In: Goldberger ME, Gorio A, Murray M, editors. Development and Plasticity of the Mammalian Spinal Cord. Padova: Liviana Press; 1986. pp. 65–80. [Google Scholar]
- Thor KB, Roppolo JR, de Groat WC. Naloxone induced micturition in unanesthetized paraplegic cats. J. Urol. 1983;129:202–205. doi: 10.1016/s0022-5347(17)51984-9. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Neurochemical plasticity and the role of neurotrophic factors in bladder reflex pathways after spinal cord injury. Prog. Brain Res. 2006;152:97–115. doi: 10.1016/S0079-6123(05)52007-7. [DOI] [PubMed] [Google Scholar]
- Vodusek DB, Light JK, Libby JM. Detrusor inhibition induced by stimulation of pudendal nerve afferents. Neurourol. Urodyn. 1986;5:381–389. [Google Scholar]
- Vodusek DB, Plevnik S, Vrtacnik P, Janez J. Detrusor inhibition on selective pudendal nerve stimulation in the perineum. Neurourol. Urodyn. 1988;6:389–393. [Google Scholar]
- Walter JS, Wheeler JS, Robinson CJ, Wurster RD. Inhibiting the hyperreflexic bladder with electrical stimulation in a spinal animal model. Neurourol. Urodyn. 1993;12:241–253. doi: 10.1002/nau.1930120306. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu H, Shen B, Roppolo JR, de Groat WC, Tai C. Bladder inhibition or excitation by electrical perianal stimulation in a cat model of chronic spinal cord injury. BJU Int. 2008;103:530–536. doi: 10.1111/j.1464-410X.2008.08029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler JS, Walter JS, Zaszczurynski PJ. Bladder inhibition by penile nerve stimulation in spinal cord injury patients. J. Urol. 1992;147:100–103. doi: 10.1016/s0022-5347(17)37145-8. [DOI] [PubMed] [Google Scholar]